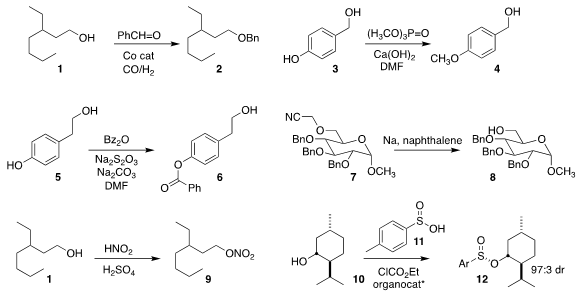
Elena V. PMID:32472497 5-Bromo-1,3-thiazole-2-carbaldehyde supplier Gusvskaya of the Universidade Federal de Minas Gerais and Matthias
Beller of the Leibniz-Institut für Katalyse e.V. used benzaldehyde to convert
the alcohol 1 to the
benzyl ether 2
(Chem. Eur. J. 2022, 28, e202103903.
DOI: 10.1002/chem.202103903).
Yu Tang and Biao Yu of the Shanghai Institute of Organic Chemistry selectively
converted the diol 3 to the methyl ether 4
(Synthesis 2022, 54, 2373.
DOI: 10.1055/a-1731-3852).
Chien-Fu Lian of the National Chung Hsing University achieved similar selectivity in
converting the diol 5 to the
monobenzoate 6
(Org. Lett. 2022, 24, 4207.
DOI: 10.1021/acs.orglett.2c01467).
Rima Thakur of the National Institute of Technology Patna showed that the cyanomethyl
ether of 7 could be deprotected to the alcohol 8, leaving the benzyl ethers intact
(Org. Biomol. Formula of 92885-03-5 Chem. 2022, 20, 4030.
DOI: 10.1039/D2OB00338D).
Le-Wu Zhan and Bin-dong Li of the Nanjing University of Science and
Technology devised an easily-scaled protocol for converting the alcohol 1 to the
nitrate ester 9
(Org. Process Res. Dev. 2022, 26, 174.
DOI: 10.1021/acs.oprd.1c00388).
Using a pentadinium catalyst, Xin Zhang and Choon-Hong Tan of Nanyang Technological University
achieved high diastereoselectivity in the combination of menthol 10 with the sulfinic acid
11 to give the sulfinate ester 12
(Nature 2022, 604, 298.
DOI: 10.1038/s41586-022-04524-4).
Susumu Saito of Nagoya University assembled the
2-oxazoline 14, a protected
form of the acid and, after reduction, the aldehyde, by cyclization of the hydroxy amide 13
(J. Org. Chem. 2022, 87, 243.
DOI: 10.1021/acs.joc.1c02318).
Michael Szostak of Rutgers University, Newark and Tieqiao Chen of Hainan University used KI to promote the
transamidation of 15 with aniline to give the amide 16
(Angew. Chem. Int. Ed. 2022, 61, e202202794.
DOI: 10.1002/anie.202202794).
Thomas Scattolin of Mirati Therapeutics used
mercaptoethanol 18 to selectively deprotect the bis-urethane 17, cleaving the
Cbz group to give 19, with the
Boc group intact
(Org. Lett. 2022, 24, 3736.
DOI: 10.1021/acs.orglett.2c01410).
Dayong Sang and Juan Tian of the Jinchu University of Technology used AlI3
to debenzylate the acyl sulfonamide 20, leading to 21
(J. Org. Chem. 2022, 87, 3586.
DOI: 10.1021/acs.joc.1c03133).
Andrew J. Carnell of the University of Liverpool used an enzyme preparation
to convert the diacid 22 to the
monomethyl ester 23
(Angew. Chem. Int. Ed. 2022,
61, e202117324.
DOI: 10.1002/anie.202117324).
Meng Tang of Lanzhou University oxidized the tosylhydrazone
derived from the aldehyde 24 to the acyl hydrazide 25. On exposure to base,
25 would be converted back to the aldehyde 24, by the McFadyen-Stevens reaction
(J. Org. Chem. 2022, 87, 3845.
DOI: 10.1021/acs.joc.1c03037).
Quassin (28) was isolated from the tropical bitterwood tree Quassia amara, long
used as a medicinal plant. In preparation for a convergent assembly of 28, Sergey V. Pronin of the University of California, Irvine
epoxidized the enone
26, then heated the product to release the enone 27
(J. Am. Chem. Soc. 2022, 144, 118.
DOI: 10.1021/jacs.1c12283).