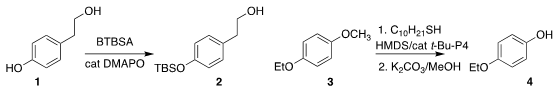
Hiroki Mandai of the Gifu University of Medical Science and Seiji Suga of
Okayama University devised conditions to selectively
protect the phenol of diol
1, leading to 2
(Chem. Lett. PMID:24455443 2022, 51, 953.
DOI: 10.1246/cl.220281).
Masanori Shigeno and Yoshinori
Kondo of Tohoku University and Toshinobu Korenaga of Iwate University
selectively deprotected the bis aryl ether 3 to give the phenol 4
(Org. Chem. BuyPrussian blue insoluble Front. 2022, 9, 3656.
DOI: 10.1039/D2QO00483F).
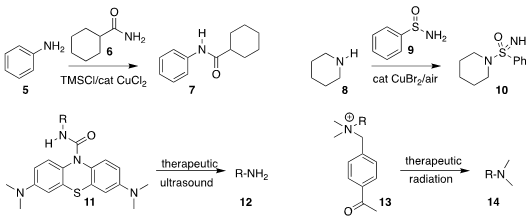
Parvesh Singh and Rajshekhar Karpoormath of the University of KwaZulu-Natal
used the amide 6 to acylate aniline 5,
leading to the amide 7
(Org. Biomol. Chem. 2022, 20, 6931.
DOI: 10.1039/D2OB01152B).
Carsten Bolm of RWTH Aachen University assembled the
NH-sulfonimidamide
10 by coupling the sulfonamide 9 with piperidine 8
(Org. Lett. 2022, 24, 6988.
DOI: 10.1021/acs.orglett.2c02804).
Xiaojun Peng of the Dalian University of Technology used
therapeutic ultrasound to liberate the free amine
12 from the urea 11
(J. Am. 912331-75-0 Purity Chem. Soc. 2022, 144, 16799.
DOI: 10.1021/jacs.2c03669).
Zhibo Liu of Peking University devised a
complementary protocol, using therapeutic radiation to
free the amine 14 from
the quaternary ammonium salt 13
(Angew. Chem. Int. Ed. 2022, 61, e202205014.
DOI: 10.1002/anie.202205014).
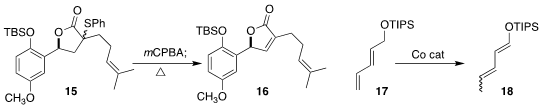
Shogo Kamo and Kazuyuki Sugita of Hoshi University showed that the
diastereomeric sulfoxides derived from the thioether 15 smoothly eliminated to
give the alkene 16
(Org. Biomol. Chem. 2022, 20, 9138.
DOI: 10.1039/D2OB01839J).
Xin Hong and Zhan Lu of Zhejiang University isomerized the dienyl ether 17 to the
silyl enol ether 18, a
protected form of the unsaturated aldehyde
(Org. Lett. 2022, 24, 4592.
DOI: 10.1021/acs.orglett.2c01701).
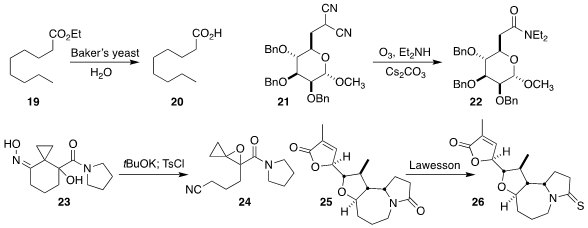
Nobuyuki Imai of the Chiba Institute of Science showed that the ester 19, having only modest water solubility, was
hydrolyzed to the free acid
20 by Baker’s yeast
(Tetrahedron Lett. 2022, 104, 154013.
DOI: 10.1016/j.tetlet.2022.154013).
Ming Li of the Ocean University of China found that the intermediate from oxidation of the bis
nitrile 21 smoothly acylated a variety of nucleophiles, including diethylamine
to give the amide 22
(Org. Lett. 2022, 24, 7944.
DOI: 10.1021/acs.orglett.2c03075).
Andrei K. Yudin of the University of Toronto opened the oxime 23
to the epoxy nitrile 24, itself a protected form of the corresponding
cyclobutanone
(Chem. Sci. 2022, 13, 12175.
DOI: 10.1039/D2SC04496J).
Huihong Wang and Zhen Wang of Lanzhou University observed that the diastereomers
of 25 were challenging to separate, but that the corresponding thioamides
26 separated readily
(Org. Chem. Front. 2022, 9, 771.
DOI: 10.1039/D1QO01578H).
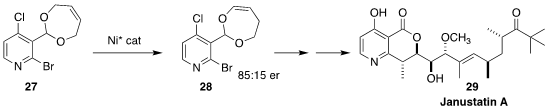
Janustatin A (29), the product of a haptophilic bacterium Gynuella sunshinyii
isolated from the Korean tidal flat sedge Carex scabrifolia, led to delayed but
synchronized cytotoxicity at nano- to picomolar concentrations. Erick N.
Carreira and Jörn Piel of the Eidgenössische Technische Hochschule Zürich
observed that conversion of the acetal of 27 into the alternative acetal 28 significantly improved the diastereoselectivity of the subsequent convergent
coupling leading to 29
(Nature Chem. 2022, 14, 1193.
DOI: 10.1038/s41557-022-01020-0).