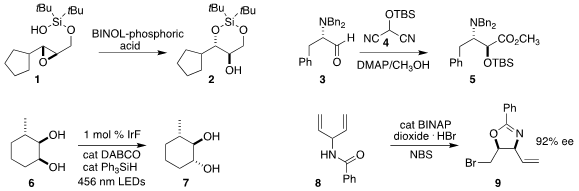
Shyam Sathyamoorthi of the University of Kansas showed that the epoxide 1 could be cyclized to the differentially-protected triol
2
(Org. Lett. 2022, 24, 939.
DOI: 10.1021/acs.orglett.1c04310).
David J. Aitken of the Université Paris-Saclay assembled the
ester 5 by
adding the protected cyanohydrin 4 to the α-amino aldehyde 3
(Org. Biomol. Chem. 2022, 20, 1769.
DOI: 10.1039/D1OB02411F).
Alison E. Wendlandt of MIT devised the photochemically-promoted
equilibration of the cis diol 6 to the trans diol 7
(J. Am. PMID:32472497 Chem. Soc. 2022, 144, 599.
DOI: 10.1021/jacs.1c11902).
David W. C. Price of 1394003-65-6 MacMillan of Princeton University
(J. Am. Cyclopropanol Order Chem. Soc. 2022, 144, 93.
DOI: 10.1021/jacs.1c11552)
and Weiping Tang of the University of Wisconsin
(J. Am. Chem. Soc. 2022, 144, 3727.
DOI: 10.1021/jacs.1c13399)
reported complentary protocols for converting trans diols to
cis diols. Yoshitaka Hamashima of the University of Shizuoka cyclized the
prochiral amide 8 to the bromide 9
(J. Am. Chem. Soc. 2022, 144, 3913.
DOI: 10.1021/jacs.1c11816).
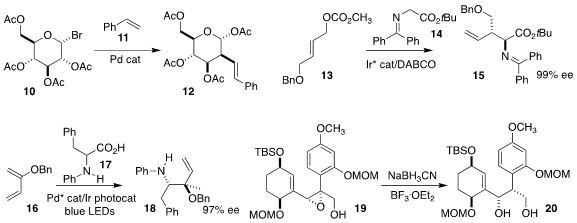
Peng Liu of the University of Pittsburgh and Ming-Yu Ngai of the State
University of New York, Stony Brook coupled the glycosyl bromide 10 with styrene
11 to give the alkene 12
(J. Am. Chem. Soc. 2022, 144, 3353.
DOI: 10.1021/jacs.1c13299).
Xiaolei Wang of Lanzhou University alkylated the protected glycine ester 14 with the allylic
carbonate 13 to give the branched product 15
(Synlett 2022, 33, 655.
DOI: 10.1055/s-0040-1719909).
Jun Zheng and Bernhard Breit of the Albert-Ludwigs-Universität Freiburg devised the
photochemically-promoted addition of the α-amino acid derivative 17 to the diene
16, leading to the tertiary ether 18
(Angew. Chem. Int. Ed. 2022, 61, e202200105.
DOI: 10.1002/anie.202200105).
Yuichiro Kawamoto and Hisanaka Ito of the Tokyo University of
Pharmacy and Life Sciences observed that
reduction of the epoxide 19 proceeded
with clean inversion, to give the diol 20
(Tetrahedron 2022, 106-107, 132595.
DOI: 10.1016/j.tet.2021.132595).
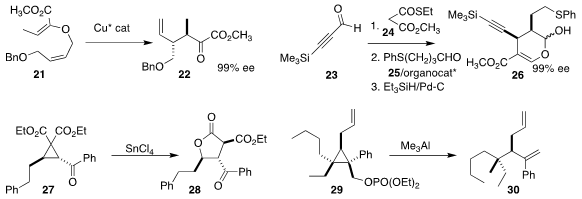
Martin Hiersemann of TU Dortmund used a Cu catalyst to rearrange the alkenyl
ether 21 to the α-keto ester 22
(Chem. Eur. J. 2022, 28, e202103558.
DOI: 10.1002/chem.202103558).
Hayato Ishikawa of Chiba University used the Jørgenson-Hayashi catalyst to mediate the
three-component coupling of the alkynyl aldehyde 23, the thioester 24 and the
aldehyde 25 to give the ester 26
(Chem. Eur. J. 2022, 28, e202104052.
DOI: 10.1002/chem.202104052).
Kannupal Srinivasan of Barathidasan University observed clean stereocontrol in the
rearrangement of the cyclopropane 27 to the
lactone 28
(Org. Biomol. Chem. 2022, 20, 3145.
DOI: 10.1039/D2OB00292B).
Ilan Marek of Technion-Israel Institute of Technology established the
acyclic quaternary center of the diene 30 by opening the cyclopropane 29
(J. Am. Chem. Soc. 2022, 144, 7066.
DOI: 10.1021/jacs.2c01695).
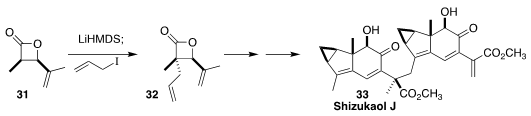
The dimeric sesquiterpenoid shizukaol J (33) was isolated from the herb
Chloranthus fortunei, used in Chinese folk medicine for the treatment of bone
fractures. Early in a synthesis of 33, Bo Liu of Sichuan University demonstrated
the efficient construction of 32 by alkylation of the β-lactone 31
(Angew. Chem. Int. Ed. 2022, 61, e202200258.
DOI: 10.1002/anie.202200258).