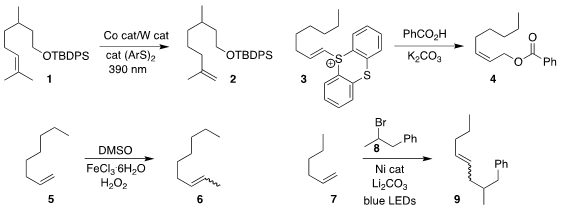
Alison E. Buy103128-76-3 Wendlandt of MIT devised a protocol for the contrathermodynamic
isomerization of the alkene
1 to the alkene 2
(J. Am. Chem. Soc. 2022, 144, 145.
DOI: 10.1021/jacs.1c12043).
Robert R. Knowles of Princeton University
(J. Am. Chem. Soc. 2022, 144, 137.
DOI: 10.1021/jacs.1c11681)
and John F. 186446-26-4 Formula Hartwig of the University of California, Berkeley
(Org. Lett. 2022, 24, 1005.
DOI: 10.1021/acs.orglett.1c03124)
described parallel investigations. Wei Shu of the Southern
University of Science and Technology reported that the thianthrenium salt 3,
readily prepared from the corresponding alkene, could be converted into the
allylic benzoate 4
(Chem. Sci. 2022, 13, 1003.
DOI: 10.1039/D1SC06577G).
Qiang Liu and Can-Cheng Guo of
Hunan University showed that the terminal alkene 5 could be methylated with
dimethyl sulfoxide, leading to the
alkene 6
(J. Org. Chem. 2022, 87, 7022.
DOI: 10.1021/acs.joc.2c00047).
Søren Kramer of the Technical University of Denmark and Ruben Martin of ICIQ
coupled the alkene 7 with the secondary bromide 8 to give 9
(ACS Catal. 2022, 12, 3815.
DOI: 10.1021/acscatal.2c01057). PMID:25023702
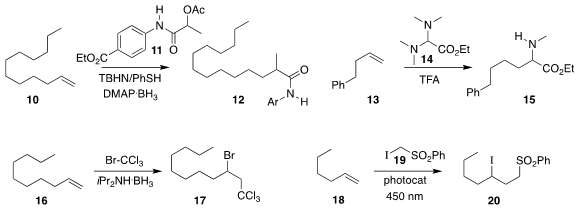
Feng-Lian Zhang, Yao Fu, and Yi-Feng Wang of the University of Science and
Technology of China assembled
the amide 12 by combining
the alkene 10 with the amide 11
(Angew. Chem. Int. Ed. 2022, 61, e202201329.
DOI: 10.1002/anie.202201329).
Nuno Maulide of the University of Vienna added the aminal 14 to the alkene
13, leading to the
α-amino ester 15
(Angew. Chem. Int. Ed. 2022, 61, e202115435.
DOI: 10.1002/anie.202115435).
Mathieu Pucheault of the University of Bordeaux showed than an amine-borane
complex was a practical radical initiator for the addition of
bromotrichloromethane to the alkene 16 to give the
bromide 17
(Chem. Commun. 2022, 58, 2124.
DOI: 10.1039/D1CC06390A).
Luca Dell’Amico of the University of Padova and Giacomo
Filippini of the University of Trieste used a photocatalyst to assemble the iodosulfone
20 by the addition of the iodosulfone 19 to the alkene 18
(ACS Catal. 2022, 12, 4290.
DOI: 10.1021/acscatal.2c00565).
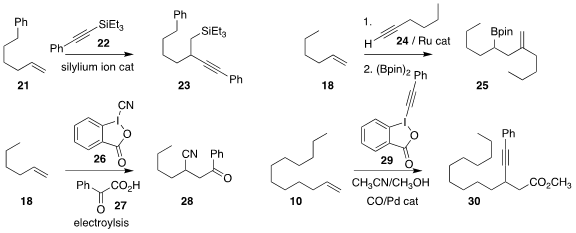
Zheng-Wang Qu of the Rheinische Friedrich-Wilhelms-Universität Bonn and Martin
Oestreich of the Technische Universität Berlin used a silylium ion to mediate the
addition of the components of the silyl alkyne 22 to the alkene 21, leading to 23
(Angew. Chem. Int. Ed. 2022, 61, e202203347.
DOI: 10.1002/anie.202203347).
Steven T. Diver of the University of
Buffalo effected cross metathesis of the alkene
18 with the alkyne 24, followed
by borylation of the resulting diene, leading to the
boronate 25
(ACS Catal. 2022, 12, 6434.
DOI: 10.1021/acscatal.2c01190).
Xianqiang Kong and Xiaohui Chen of the Changzhou Institute of
Technology and Zhong-Yan Cao of Hunan University assembled the
nitrile 28 by
electrolyzing the alkene 18, the cyanobenziodooxolone 26, and the α-keto acid 27
(J. Org. Chem. 2022, 87, 7013.
DOI: 10.1021/acs.joc.1c03134).
Pinhong Chen and Guosheng Liu of the Shanghai
Institute of Organic Chemistry used the alkynyl benziodooxolone 29 to convert
the alkene 10 to the ester 30
(Chem. Commun. 2022, 58, 2544.
DOI: 10.1039/D1CC07092D).
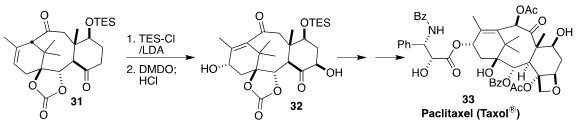
Paclitaxel (Taxol®) 33 is widely used clinically as an anti-cancer agent. In
the course of a synthesis of 33, Noritaka Chida of Keio University showed that
DMDO was particularly selective in the
epoxidation of the bis enol ether derived
from 31, leading to the diol 32
(Org. Lett. 2022, 24, 202.
DOI: 10.1021/acs.orglett.1c03851).
For a practical preparation of DMDO, see Org. Synth. 2013, 90, 350. (DOI: 10.15227/orgsyn.090.0350)