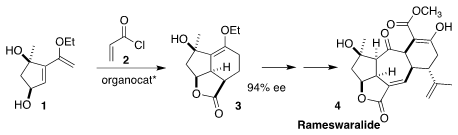
Rameswaralide (4), isolated from the soft coral Sinularia dissecta,
showed modest cytotoxicity. 2411793-14-9 supplier In the course of a synthesis of 4, Daniel
Romo of Baylor University showed that with an enantiomerically-pure
benzotetramisole catalyst, acryloyl chloride 2 reacted with only one
enantiomer of the diene 1, leading to 3 in high ee
(J. Am. Chem. Soc. PMID:34816786 2022, 144, 18575.
DOI: 10.1021/jacs.2c08245).
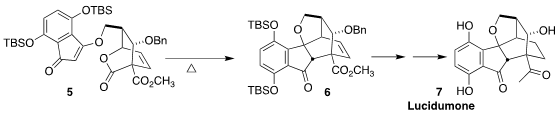
Lucidumone (7), isolated from the mushroom Ganoderma lucidum,
selectively inhibits COX-2. Aurélien de la Torre of the Université Paris-Saclay
constructed the 6, simply converted to 7, by the
retro-Diels-Alder/Diels-Alder cyclization of the lactone 5
(J. Am. Chem. Price of Benzaldoxime Soc. 2022, 144, 17803.
DOI: 10.1021/jacs.2c08760).
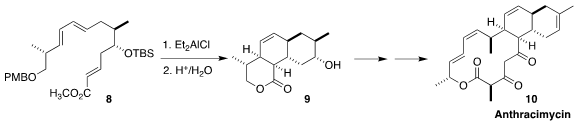
Anthracimycin (10), isolated from a marine Streptomyces species
(CNH365), showed potent activity against Gram-positive bacteria, including MRSA
and anthrax. Pei-Yuan Qian and Rongbiao Tong of the Hong Kong University of
Science and Technology devised a concise route to 10, based on the
cyclization of the triene 8 to the tricyclic
lactone 9
(Chem. Sci. 2022, 13, 12776.
DOI: 10.1039/D2SC05049H).
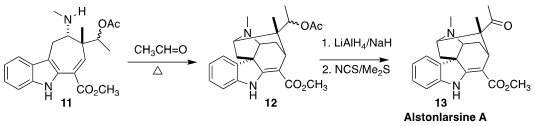
Alstonlarsine A (13), isolated from Alstonia scholaris, the "scholar
tree" of Southeast Asia, was found to be an inhibitor of DAP kinase-related
apoptosis-inducing protein kinase 2 (Drak2), a signaling molecule involved in
the regulation of inappropriate T cell activation. Zorana Ferjancic and Filip
Bihelovic of the University of Belgrade combined the secondary amine 11
with acetaldehyde, leading to an enamine that cyclized to 12. Selective
reduction of the acetate followed by oxidation led to 13
(Angew. Chem. Int. Ed. 2022, 61, e202210297.
DOI: 10.1002/anie.202210297).
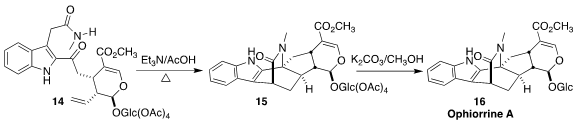
Ophiorrine A (16) was isolated from the Chinese medicinal plant
Ophiorrhiza japonica. Following the likely biosynthesis, Guillaume Vincent, also
of the Université Paris-Saclay, assembled 16 by the cyclodehydration of
the amide 14, followed by in situ cyclization to 15
(Angew. Chem. Int. Ed. 2022, 61, e202209135.
DOI: 10.1002/anie.202209135).
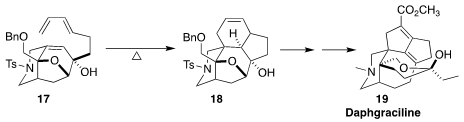
The yuzurine alkaloid daphgraciline (19) was isolated from the
evergreen Daphniphyllum gracile Gage of New Guinea. Jianwei Sun, also of
the Hong Kong University of Science and Technology, and Chuang-Chuang Li of the
Southern University of Science and Technology constructed the tetracyclic core
18 of 19 by the cyclization of the triene 17
(J. Am. Chem. Soc. 2022, 144, 18823.
DOI: 10.1021/jacs.2c09548).