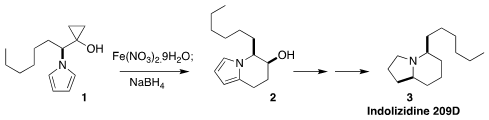
Indolizidine 209D (3), isolated from the neotropical "poison dart"
frog, inhibits neuromuscular transmission. Hanyue Qiu, Ling He and Min Zhang of
Chongqing University rearranged the cyclopropanol 1, prepared from the
corresponding ester by the
Kulinkovich reaction,
to a ketone that was reduced to the alcohol 2. Further reduction led to 3
(Org. Lett. 2023, 25, 2058.
DOI: 10.1021/acs.orglett.3c00406). 3-Bromo-2-iodobenzo[b]thiophene Formula
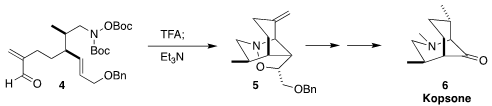
Kopsone (6) was isolated from the Malaysian shrub Kopsia profunda. 6-Bromo-8-fluoroisoquinolin-1(2h)-one Price
En route to 6, Satoshi Yokoshima of Nagoya University cyclized the
unsaturated aldehyde 4 to the tricyclic 5
(Org. Lett. PMID:23291014 2023, 25, 2718.
DOI: 10.1021/acs.orglett.3c00838).
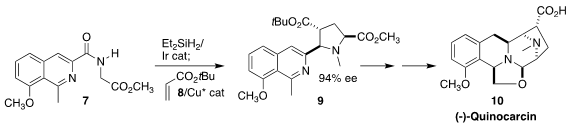
Quinocarcin (10), isolated from the culture broth of Streptomyces
melanovinaceus, showed remarkable antiproliferative activity against
lymphocytic leukemia, non-small cell lung cancer, and adenocarcinoma. Jian-Feng
Zheng and Pei-Qiang Huang of Xiamen University secured the absolute
configuration of 10 by the
reduction of the amide 7, followed by
Cu-catalyzed dipolar
cycloaddition to t-butyl acrylate 8 to give 9
(Angew. Chem. Int. Ed. 2023, 62, e202302832.
DOI: 10.1002/anie.202302832).
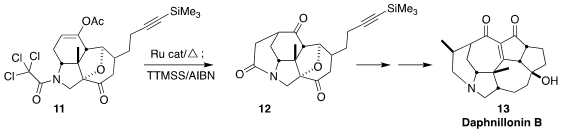
Daphnillonin B (13) was isolated from the tropical tree
Daphniphyllum longeracemosa. Chuang-Chuang Li of the Southern University of
Science and Technology constructed the
piperidine of 13 by the
Ru-catalyzed cyclization of 11 to 12, followed by further reduction
(J. Am. Chem. Soc. 2023, 145, 10998.
DOI: 10.1021/jacs.3c03755).
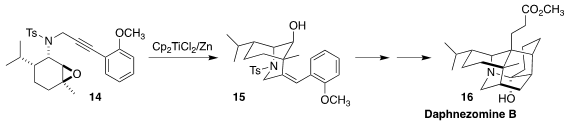
Daphnezomine B (16), isolated from the leaves of the East Asian shrub
Daphniphyllum humile, showed cytotoxicity toward murine lymphoma L1210
cells. Hongbin Zhai of the Shenzhen Graduate School of Peking University
assembled the piperidine ring of 16 by the one-electron reduction and
cyclization of the piperitone-derived epoxide 14 to 15
(Angew. Chem. Int. Ed. 2023, 62, e202303402.
DOI: 10.1002/anie.202303402).
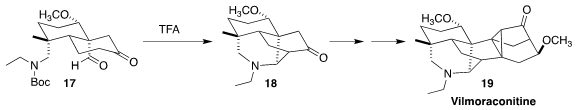
Vilmoraconitine (19) was isolated from the herb Aconitum
vilmorinianum, used in traditional Chinese medicine to treat rheumatism and
pains. Xiao-Yu Liu and Yong Qin of the West China School of Pharmacy assembled
the key tetracyclic intermediate 18 by the intramolecular Mannich
cyclization of the keto aldehyde 17
(J. Am. Chem. Soc. 2023, 145, 3903.
DOI: 10.1021/jacs.3c00318).