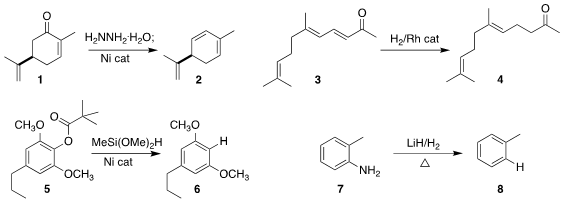
Liang-Nian He of Nankai University and Chao-Jun Li of McGill University
converted the hydrazone of carvone 1 to the triene 2
(JACS Au 2022, 2, 1929.
DOI: 10.1021/jacsau.2c00320).
Mathias Schelwies of BASF Ludwigshafen and Rylan L. PMID:23341580 Lundgren of the University
of Alberta selectively
reduced the enone
3 to the ketone 4
(Angew. Chem. Int. Ed. Formula of 197632-76-1 2022, 61, e202210601.
DOI: 10.1002/anie.202210601).
Bert U. W. Maes of the University of Antwerp reduced the aryl ester 5
to the arene 6
(Angew. Chem. Int. Ed. 2022, 61, e202201751.
DOI: 10.1002/anie.202201751).
Jianping Guo and Ping Chen of the Dalian Institute of Chemical Physics and Anan
Wu of Xiamen University reduced
o-toluidine 7 to toluene 8
(J. Am. 2-(4-Ethynylphenyl)acetic acid Order Chem. Soc. 2022, 144, 17441.
DOI: 10.1021/jacs.2c05586).
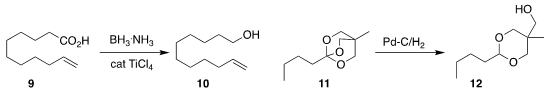
P. Veeraraghavan Ramachandran of Purdue University used TiCl4 to catalyze the
borane
reduction of the carboxylic acid
9 to the alcohol 10
(Org. Lett. 2022, 24, 8481.
DOI: 10.1021/acs.orglett.2c03326).
Renat Kadyrov of the Academy of Sciences of the Czech Republic
hydrogenated the orthoester 11 to the acetal 12
(J. Org. Chem. 2022, 87, 12673.
DOI: 10.1021/acs.joc.2c01116).
Liam J. Donnelly and Thibault Canat of the Université Paris-Saclay used
Schwartz’s reagent as a catalyst for the
reduction of the amide
13 to the imine 14
(Angew. Chem. Int. Ed. 2022, 61, e202206170.
DOI: 10.1002/anie.202206170).
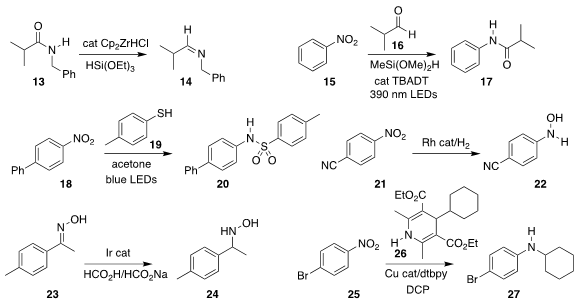
Mulian Zhang and Jie Wu of the
National University of Singapore assembled the amide 17 by coupling the
nitroarene 15 with the aldehyde 16
(Chem. Sci. 2022, 13, 9361.
DOI: 10.1039/D2SC03047K).
Shi-Chao Ren and Yonggui Robin Chi of Nanyang Technological University prepared the sulfonamide
20 by combining the nitroarene 18 with the thiophenol 19
(Org. Lett. 2022, 24, 8907.
DOI: 10.1021/acs.orglett.2c03770).
Christophe Werlé of the Max Planck Institute for Chemical Energy Conversion
hydrogenated the nitroarene 21 to the hydroxylamine 22
(Angew. Chem. Int. Ed. 2022, 61, e202205515.
DOI: 10.1002/anie.202205515).
Lu Ouyang and Renshi Luo of Gannan Medical University
used formate to reduced the oxime 23 to the hydroxylamine 24
(Org. Biomol. Chem. 2022, 20, 6394.
DOI: 10.1039/D2OB01084D).
Xuan-Hui Ouyang and Ren-Jie Song of Nanchang Hangkong
University and Jin-Heng Li of Henan Normal University alkylated the nitroarene
25 with the Hantzsch ester 26, leading to the coupled amine 27
(Org. Chem. Front. 2022, 9, 4070.
DOI: 10.1039/D2QO00706A).
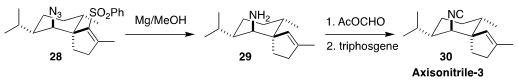
Axisonitrile-3 (30), isolated from the marine sponge Axinella cannabina,
exhibited potent anti-fouling activity. In the course of a synthesis of 30, Yoshiyasu Ichikawa of Kochi University and Seijiro Hosokawa of Waseda University
showed that Mg in methanol was effective both for the desulfonylation of the
unsaturated sulfone 28, and for the concomitant reduction of the azide to the
amine, that was readily carried on to the isonitrile
(Org. Biomol. Chem. 2022, 20, 8236.
DOI: 10.1039/D2OB00486K).