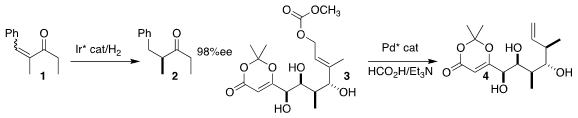
Pher G. Andersson of Stockholm University showed that a Z/E
mixture of the enone 1 could be hydrogenated to 2 in high ee
(Nature Commun. 2022, 13, 361.
DOI: 10.1038/s41467-022-28003-6).
Michael J. PMID:34235739 Price of 1217725-33-1 Krische of the University of Texas effected the
enantioselective Tsuji reduction of the allylic carbonate 3, leading to the
alkene 4 as a single diastereomer
(J. Am. Chem. Soc. 4-Oxotetrahydrofuran-3-carbonitrile custom synthesis 2022, 144, 1016.
DOI: 10.1021/jacs.1c12063).
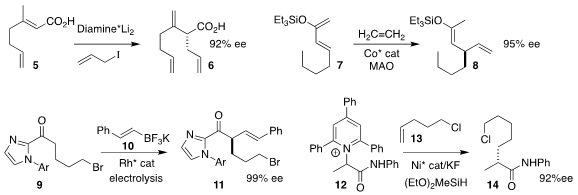
Houhua Li of Peking University used a diamine to direct the absolute course
of the allylation of the unsaturated carboxylic acid 5, leading the
disubstituted alkene 6
(Angew. Chem. Int. Ed. 2022, 61, e202116136.
DOI: 10.1002/anie.202116136).
T. V. RajanBabu of the Ohio State University assembled the
diene 8 by coupling the
enol silyl ether 7 with ethylene
(ACS Catal. 2022, 12, 5094.
DOI: 10.1021/acscatal.2c00546).
Eric Meggers of Philips-Universität Marburg used electrolysis to effect the coupling of the acyl
imidazole 9 with the alkenyl borane 10, delivering 11
(J. Am. Chem. Soc. 2022, 144, 6964.
DOI: 10.1021/jacs.2c01686).
Ming Shang of Shanghai Jiao Tong University showed that the Katritzky pyridinium salt
12 could be coupled with the terminal alkene 13 to give the
amide 14
(J. Am. Chem. Soc. 2022, 144, 1130.
DOI: 10.1021/jacs.1c12350).
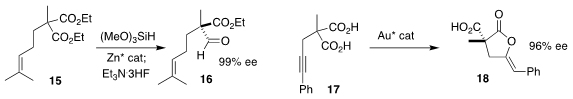
Zhongxing Huang of the University of Hong Kong reduced the prochiral diester
15 to the aldehyde 16 in high ee
(J. Am. Chem. Soc. 2022, 144, 6918.
DOI: 10.1021/jacs.2c01380).
Gen-Qiang Chen and Xumu Zhang of the Southern University of Science and Technology and
Chi-Ming Che, also of the University of Hong Kong, used a gold catalyst to
cyclize the diacid 17 to the
lactone 18
(Angew. Chem. Int. Ed. 2022, 61, e202201739.
DOI: 10.1002/anie.202201739).
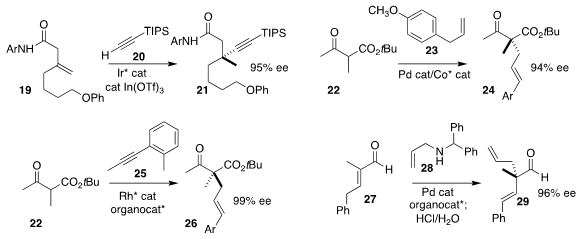
Bi-Jie Li of Tsinghua University coupled the alkyne 20 with the alkene
19, leading to the amide 21
(Angew. Chem. Int. Ed. 2022, 61, e202201099.
DOI: 10.1002/anie.202201099).
Yangbin Liu of Shenzhen Bay Laboratory and Xiaoming Feng of Sichuan University effected
the oxidative coupling of the β-ketoester 22 with the allylbenzene derivative
23, to give 24
(Angew. Chem. Int. Ed. 2022, 61, e202115715.
DOI: 10.1002/anie.202115715).
Xueling Mi of Beijing Normal University used an enantiomerically-pure diamine to mediate the
coupling of 22 with the alkyne 25, to deliver 26
(Org. Lett. 2022, 24, 1186.
DOI: 10.1021/acs.orglett.1c04334).
Stacey E. Brenner-Moyer of Rutgers/Newark found that a BINOL-derived phosphoric
acid effectively directed the alkylation of the unsaturated aldehyde 27 with the
allylic amine 28, leading, after hydrolysis, to 29
(J. Org. Chem. 2022, 87, 866.
DOI: 10.1021/acs.joc.1c02591).
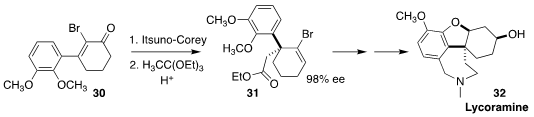
Lycoramine (32), isolated from the red spider lily Lycoris radiata, is a potent
acetylcholinesterase (AChE) inhibitor. Alakesh Bisai of the Indian Institute of
Science Education and Research Kolkata established the cyclic quaternary center
of 31 and thus 32 by Itsuno-Corey reduction of the cyclohexenone
30 followed by
Johnson-Claisen rearrangement
(J. Org. Chem. 2022, 87, 7786.
DOI: 10.1021/acs.joc.2c00420).