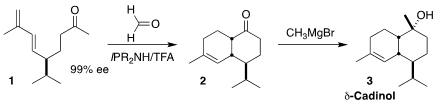
Stefan Schulz of the Technische Universität Braunschweig updated the classic
intramolecular Diels-Alder cycloaddition of the triene 1 to the ketone
2, readily convertible to δ-cadinol (3), by preparing 1 via an
enantioselective Michael addition, and carrying it on to 2 via cascade
enone formation/cyclization. δ-Cadinol (3) is also known as torreyol,
sesquigoyol, albicaulol and pilgerol
(Beilstein J. Fmoc-O-Methyl-L-Homoseri web Org. 6-Methoxy-5-nitropicolinic acid site Chem. 2023, 19, 167.
DOI: 10.3762/bjoc.19.16). PMID:24025603
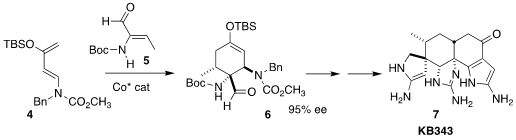
The pentacyclic guanidinium alklaloid KB343 (7) was isolated off the
coast of Palau from the tunicate Epizoanthus illoricatus. Phil S. Baran
assembled 7 by way of the enantioselective Co-catalyzed cycloaddition of
the unsaturated aldehyde 5 with Rawal’s diene 4 to give 6
(J. Am. Chem. Soc. 2023, 145, 7753.
DOI: 10.1021/jacs.3c01991).
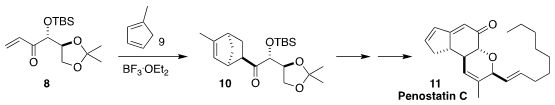
Methyl cyclopentadiene 9 is an equilibrating mixture of three
regioisomers. Nevertheless, Xiaochuan Chen of Sichuan University showed that
cycloaddition with the enone 8 proceeded via the most reactive of the
three, leading to the ketone 10, that they carried on to penostatin C (11)
(Org. Lett. 2023, 25, 1941.
DOI: 10.1021/acs.orglett.3c00485).
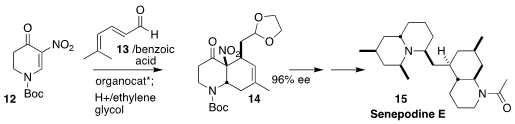
The aldehyde 13 is not a very reactive Diels-Alder diene. Hayato
Ishikawa of Chiba University showed that in the presence of benzoic acid, 13
was in equilibrium with the corresponding β-,γ-isomer, which engaged with the
dienophile 12 to give 14, the carbocyclic portion of senopodine E (15)
(Org. Lett. 2023, 25, 1151.
DOI: 10.1021/acs.orglett.3c00133).
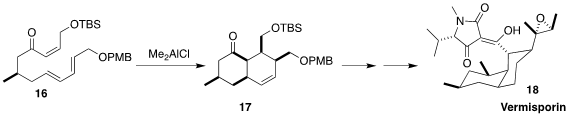
As Xiaoguang Lei of Peking University anticipated, Diels-Alder cyclization of
the Z-enone 16, although reluctant, could be accelerated with Lewis acid,
proceeding via an ordered boat transition state to give the cis-fused ketone 17.
This was carried on to the antibiotic vermisporin (18), originally isolated from
the fungus Ophiobolus vermisporus L-8
(Angew. Chem. Int. Ed. 2023, 62, e202301872.
DOI: 10.1002/anie.202301872).
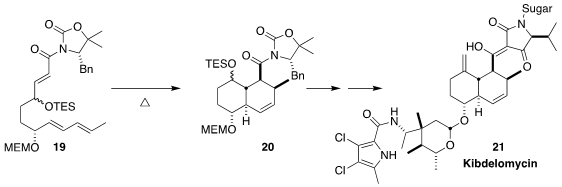
The triene 19 was prepared by Rainer Schobert as an inconsequential mixture
of epimers. The diastereoselective Diels-Alder cyclization of the E-enone to 20
was facile, setting the stage for the preparation of a known advanced
intermediate in the synthesis of the antibiotic kibdelomycin (21)
(Chem. Sci. 2023, 14, 3562.
DOI: 10.1039/D3SC00595J).