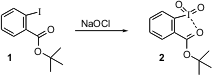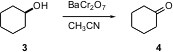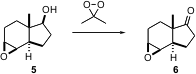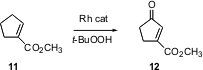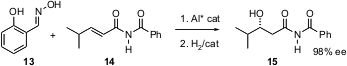Efficient new methods for oxidation are always welcome. The Dess-Martin periodinane has become the workhorse for alcohol to aldehyde or ketone conversion in organic research labs around the world. 5-Azaspiro[2.5]octane-6,8-dione Chemscene 219640-94-5 In stock Viktor V. Zhdankin of the University of Minnesota, Duluth has described (Chem. Commun. 2004, 106. DOI: 10.1039/b312961f)a complementary family of reagents. PMID:24455443 Oxidation of an ester 1 ofo-iodobenzoic acid withNaOCl delivers 2. Depending on the ester, the reagent 2 is soluble and an effective oxidant, with KBr catalysis, in a wide range of organic solvents. Presumably, the spent oxidant can be recovered and recycled.
Chromium-based oxidants, noteworthy for their specificity and ease of use, continue to be popular. Enayatollah Mottaghinejad of Azad University of Iran, Tehran has found (Tetrahedron Lett. 2004, 45, 8823. DOI: 10.1016/j.tetlet.2004.10.010)that barium dichromate, easily prepared, smoothly oxidizes alcohols to aldehydes and ketones in refluxing acetonitrile.
A recent report (J. Org. Chem. 2004, 69, 8510. DOI: 10.1021/jo048816w)by Paul G. Williard of Brown University and Ruggero Curci of Università di Bara of the oxidation of 5 to 6 serves as a timely reminder that the widely-used epoxidation reagent dimethyl dioxirane is also useful for the oxidation of secondary alcohols to ketones.
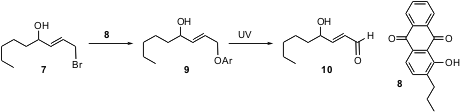
α,β-Unsaturated aldehydes can also be prepared by the net oxidation of allylic halides. Paul B. Jones of Wake Forest University has put forward (Org. Lett. 2004, 6, 3767. DOI: 10.1021/ol048478l)an elegant new method for effecting this transformation, based on nucleophilic displacement by the phenol 8. Photolysis of the stable aryl ether 9 delivers the unstable aldehyde 10.
Allylic oxidation offers another route to ketones. Michael P. Doyle of the University of Maryland has found (J. Am. Chem. Soc. 2004, 126, 13622. DOI: 10.1021/ja045330o)that Rh caprolactam is a very active (0.1 mol %) catalyst for this conversion.
Although the transformation is not an oxidation, Eric N. Jacobsen of Harvard University has developed (J. Am. Chem. Soc. 2004, 126, 14724.DOI: 10.1021/ja045563f)an elegant route to enantiomerically-pure alcohols, based on the conjugate addition of an oxime such as 13 to an α,β-unsaturated imide such as 14.