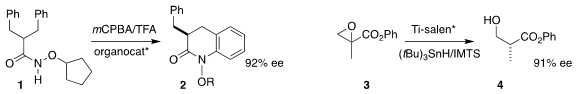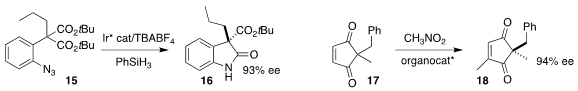Xufeng Lin of Zhejiang University used an enantiomerically-pure bis iodide to
mediate the oxidative cyclization of the prochiral amine 1 to the
lactam
2
(Tetrahedron Lett. 2022, 109, 154157.
DOI: 10.1016/j.tetlet.2022.154157).
Yong-Qiang Zhang of Shandong University achieved high ee in the
reduction of
the racemic epoxide 3 to the primary alcohol 4
(Angew. Chem. Int. PMID:28440459 Ed. Buy170853-04-0 2022, 61, e202214111.
DOI: 10.1002/anie.202214111).
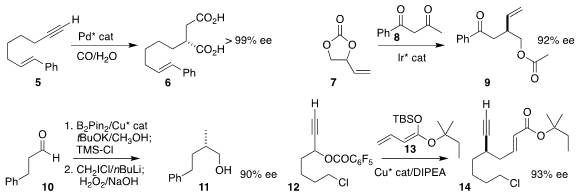
Kaiwu Dong of East China Normal University used Pd relay catalysis to convert
the terminal alkyne 5 to the
diacid 6
(Angew. Chem. Int. Ed. 2022, 61, e202204156.
DOI: 10.1002/anie.202204156).
Xiu-Qin Dong and Chun-Jiang Wang of Wuhan University assembled the
acetate 9 by coupling the carbonate 7 with the diketone 8
(J. Am. Chem. Soc. 2022, 144, 20025.
DOI: 10.1021/jacs.2c08811).
Tao Xu of Tongji University effected enantioselective
conversion of the aldehyde 10 to the α-chloroborane, leading via application of
the Matteson protocol to the primary alcohol 11
(J. Am. Chem. Soc. 2022, 144, 22870.
DOI: 10.1021/jacs.2c11024).
Guoyin Yin of Wuhan University reported a parallel procedure
(not illustrated) beginning with a terminal alkene
(Angew. 791616-62-1 web Chem. Int. Ed. 2022, 61, e202209076.
DOI: 10.1002/anie.202209076).
Hao Xu of Central China Normal University prepared the alkyne 14 by
adding the ketene silyl acetal 13 to the racemic propargylic acetate 12
(J. Am. Chem. Soc. 2022, 144, 15779.
DOI: 10.1021/jacs.2c06560).
Peng Liu of the University of Pittsburgh and Ohyun Kwon of UCLA cyclized the
prochiral malonate 15 to the
oxindole 16
(J. Am. Chem. Soc. 2022, 144, 21318.
DOI: 10.1021/jacs.2c09421).
Santanu Mukherjee of the Indian Institute of Science, Bangalore and Jennifer S.
Hirschi of Binghamton University elucidated
(J. Am. Chem. Soc. 2022, 144, 17399.
DOI: 10.1021/jacs.2c02941) the
mechanism of the previously reported
(J. Am. Chem. Soc. 2015, 137, 130.
DOI: 10.1021/ja5117556)
Cinchona-mediated desymmetrizing addition of nitromethane to the prochiral
enedione 17, leading to the methylated 18 in high ee.
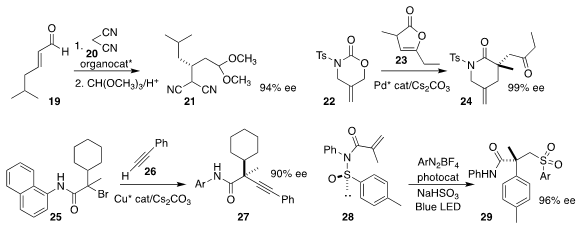
Yujiro Hayashi of Tohoku University used a diarylprolinol-derived catalyst to
direct the addition of malononitrile 20 to the unsaturated aldehyde 19, leading
after protection to the acetal 21
(Synlett 2022, 33, 1831.
DOI: 10.1055/a-1846-5007).
Ming-Qing Zhou and Wei-Cheng Yuan of Chengdu University constructed the
lactam
24 by coupling the
urethane 22 with the lactone 23
(Org. Lett. 2022, 24, 8348.
DOI: 10.1021/acs.orglett.2c03383).
Qiang-Shuai Gu and Xin-Yuan Liu of the Southern University of Science and Technology and Xin Hong,
also of Zhejiang University, coupled the alkyne 26 with the racemic α-bromo
amide 25, to give the alkyne 27 in high ee
(Nature Chem. 2022, 14, 949.
DOI: 10.1038/s41557-022-00954-9).
Shaoyu Li of Taizhou University, Zhiyuan Chen of Zhejiang University City College, and
Jie Wu of Fudan University prepared the sulfone 29 by adding an arenediazonium
salt to the N-arylsulfinyl acrylamide 28
(Nature Commun. 2022, 13, 7081.
DOI: 10.1038/s41467-022-34836-y).
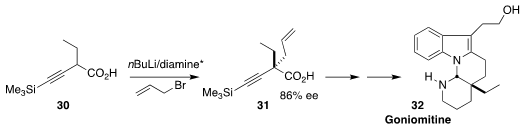
The alkaloid goniomitine 32, isolated from Gonioma malagasy, shows nanomolar
antiproliferative activity against several human tumor cell lines. Armen
Zakarian of the University of California, Santa Barbara established the central
quaternary center of 32 by the chiral amine-mediated
allylation of the acid
30 to give 31
(Angew. Chem. Int. Ed. 2022, 61, e202209987.
DOI: 10.1002/anie.202209987).