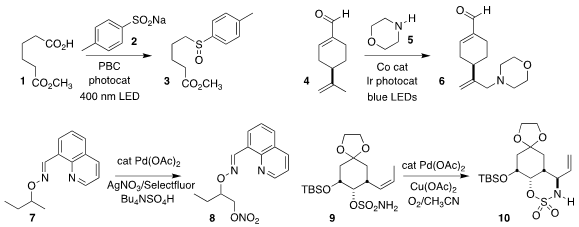
Oleg V. Larionov of the University of Texas at San Antonio devised a protocol
for the decarboxylative coupling of an acid 1 with an arenesulfinate 2 to give
the sulfoxide 3
(Angew. Chem. Int. Ed. 2022, 61, e202210525.
DOI: 10.1002/anie.202210525).
Xiaotian Qi and Aiwen Lei of Wuhan University effected the coupling of the alkene 4 with the
amine 5, leading to the
allylic amine 6
(Nature Catal. Formula of Methyl 4-chloro-3-oxobutanoate 2022, 5, 642.
DOI: 10.1038/s41929-022-00818-y).
Jun Luo, Bing-Cheng Hu, and Chao Jiang of the Nanjing University of Science and
Technology oxidized the oxime ether 7 to the
nitrate ester 8, that could be
reduced to the diol or amine, or directly converted to the corresponding azide
(Org. Biomol. Chem. PMID:24818938 2023, 21, 75.
DOI: 10.1039/D2OB01919A).
Shyam Sathyamoorthi of the University of
Kansas cyclized the sulfamate 9 to the allylic amine 10
(Tetrahedron 2022, 128, 133112.
DOI: 10.1016/j.tet.2022.133112).
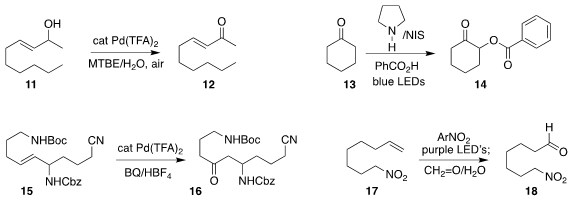
Zhenbo Gao of Nanjing Agricultural University developed mild conditions for
the selective oxidation of an allylic alcohol 11 to the
enone 12
(Tetrahedron Lett. Price of 5-Bromo-4-chloropicolinic acid 2022, 103, 153976.
DOI: 10.1016/j.tetlet.2022.153976).
John C.-G. Zhao, also of the University of Texas at San Antonio, effected the
α-acyloxylation
of the ketone 13 to the ester 14
(Chem. Commun. 2022, 58, 11308.
DOI: 10.1039/D2CC04016F).
Sebastian Stecko of the Polish Academy of
Sciences observed high regioselectivity in the
Wacker oxidation of the allylic
amine 15 to the ketone 16
(Org. Biomol. Chem. 2023, 21, 115.
DOI: 10.1039/D2OB01843H).
Marco Simonetti of the University of Manchester and Daniele Leonori of RWTH Aachen University
devised a practical protocol for the nitroarene-mediated
cleavage of the alkene
17 to the aldehyde 18
(Nature 2022, 610, 81.
DOI: 10.1038/s41586-022-05211-0).
Marvin Parasram of New York University described in more detail the historical background of this cleavage
(J. Am. Chem. Soc. 2022, 144, 15437.
DOI: 10.1021/jacs.2c05648).
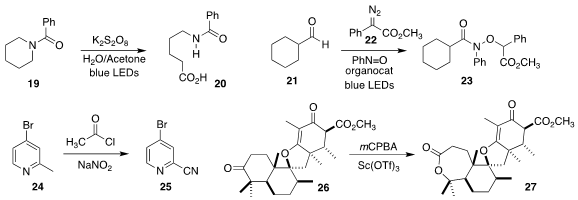
Maciej Giedyk, also of the Polish Academy of Sciences, reported the oxidation
of the amide 19 to the acid 20
(Eur. J. Org. Chem. 2022, e202200913.
DOI: 10.1002/ejoc.202200913).
Rene M. Koenigs, also of RWTH Aachen University, and Jun Xuan of Anhui University
assembled the amide 23 by the triazolium salt-catalyzed coupling of the aldehyde
21 and the diazo ester 22 with nitrosobenzene
(ACS Catal. 2022, 12, 11129.
DOI: 10.1021/acscatal.2c02875).
Yu Yuan of Yangzhou University used
sodium nitrite to oxidize the methyl pyridine
24 to the nitrile 25
(Org. Lett. 2022, 24, 6341.
DOI: 10.1021/acs.orglett.2c02596).
John A. Porco Jr. and Feng Yang of Boston
University employed Sc(OTf)3 to promote the
Baeyer-Villiger conversion of the
ketone 26 to the lactone 27
(J. Am. Chem. Soc. 2022, 144, 12970.
DOI: 10.1021/jacs.2c05366).
Bufospirostenin A (30), isolated from the bile of the toad Asiatic toad
Bufo gargarizans, is a potent inhibitor of Na/K ATPase. In the course of a synthesis
of 30, Xin Hong of Zhejiang University and Jinghan Gui of the Shanghai Institute
of Organic Chemistry effected the oxidative cleavage of the tertiary alcohol 28
to the medium ring ketone 29
(J. Am. Chem. Soc. 2022, 144, 17769.
DOI: 10.1021/jacs.2c07944).