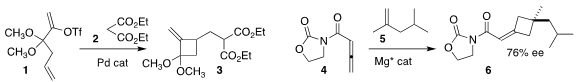
John P. Price of 3-Chloro-1H-pyrazole Wolfe of the University of Michigan constructed the
cyclobutene 3 by coupling the enol triflate
1 with diethyl malonate 2
(Org. Lett. 2022, 24, 8208.
DOI: 10.1021/acs.orglett.2c03308).
Xiaohua Liu and Xiaoming Feng of Sichuan University achieved significant enantiomeric excess in the Mg-catalyzed addition of the
allene 4 to the alkene 5 to give the cyclobutene 6
(Angew. Chem. Int. PMID:35850484 Ed. 2022, 61, e202211596.
DOI: 10.1002/anie.202211596).
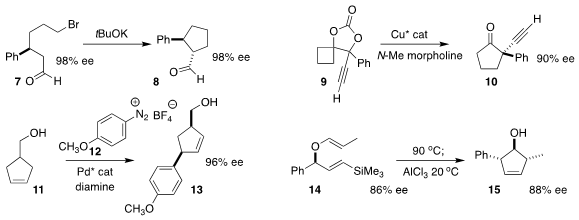
While attempted intermolecular alkylation of aldehydes often leads to C-O rather than C-C bond formation,
the cyclization of the bromoaldehyde 7 to the
cyclopentane 8 by Jean-Luc Vasse of the Université de Reims
Champagne Ardenne is a reminder that intramolecular alkylation of an aldehyde is often efficient
(Org. Biomol. (6Z,9Z)-18-Bromooctadeca-6,9-diene site Chem. 2022, 20, 5803.
DOI: 10.1039/D2OB01073A).
Xinqiang Fang of the Fujian Institute of Research on the
Structure of Matter expanded the prochiral
cyclobutane 9 to the
cyclopentanone 10 in high ee
(ACS Catal. 2022, 12, 12036.
DOI: 10.1021/acscatal.2c03623).
Abbas Hassan of Quaid-i-Azam University assembled
the cyclopentene
13 by adding the diazonium salt 12 to the prochiral cyclopentene 11
(Tetrahedron Lett. 2022, 111, 154204.
DOI: 10.1016/j.tetlet.2022.154204).
Matthew J. Cook of Montana State University used a
Claisen-Sakurai
strategy to cyclize the enol ether 14 to the cyclopentene 15
(J. Org. Chem. 2022, 87, 12250.
DOI: 10.1021/acs.joc.2c01397).
Shu-Li You of the Shanghai Institute of Organic Chemistry prepared the enone
18 by alkylating the β-naphthol 16 with the allylic carbonate 17
(Org. Lett. 2022, 24, 8031.
DOI: 10.1021/acs.orglett.2c03262).
Qing-An Chen of the Dalian Institute of Chemical Physics dimerized isoprene
19 in the presence of the heterocycle 20, leading to the
cyclohexene 21
(Nature Catal. 2022, 5, 708.
DOI: 10.1038/s41929-022-00825-z).
Zhi-Xiang Yu of Peking University effected the
ring expansion of the
cyclobutanone
22 to the
cyclohexenone 23
(J. Am. Chem. Soc. 2022, 144, 21457.
DOI: 10.1021/jacs.2c04244).
Yoshitaka Matsushima of the Tokyo University of Agriculture
observed high diastereoselectivity in the reductive cyclization of the keto aldehyde
24 to the highly-substituted
cyclohexanol 25
(Tetrahedron Lett. 2022, 106, 154060.
DOI: 10.1016/j.tetlet.2022.154060).
Professor Liu and Liming Zhang of the University of California, Santa Barbara cyclized the β-naphthol
26 to the cyclohexenone 27
(Angew. Chem. Int. Ed. 2022, 61, e202207518.
DOI: 10.1002/anie.202207518).
Ming Yang of Lanzhou University effected
the catalytic Ti(III) cyclization of the epoxide 28 to the decalin 29
(Nature Commun. 2022, 13, 6633.
DOI: 10.1038/s41467-022-34404-4).
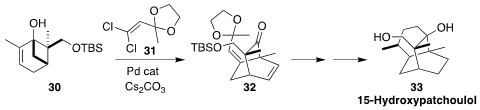
Patchoulol (“patchouli alcohol”) is a sesquiterpene alcohol found in patchouli oil,
an important material in perfumery. Richmond Sarpong of the University of California,
Berkeley devised a route to the patchoulol skeleton, leading to 15-hydroxypatchoulol
(33). A key step in the synthesis was the Pd-mediated coupling of the dichloride
31 with the tertiary alcohol 30, leading to 32. The alcohol
30 was prepared by Ti(III) cyclization of carvone epoxide
(J. Am. Chem. Soc. 2022, 144, 19253.
DOI: 10.1021/jacs.2c09201).