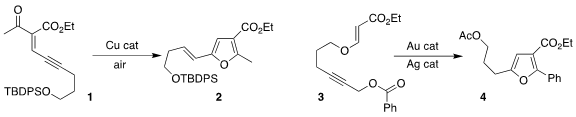
Kegong Ji of the Northwest Agricultural & Forestry University used a Cu
catalyst to cyclize the alkyne 1 to the
furan 2
(Org. 8-Hydroxyoctanoic acid In stock Chem. Front. 2023, 10, 767.
DOI: 10.1039/D2QO01741E).
Zhen Yang of Shaozing University and Huaiji Zheng, also of the
Northwest Agricultural & Forestry University, used a gold/silver catalyst
combination to cyclize the alkyne 3 to the furan 4
(J. Org. Chem. PMID:24957087 2023, 88, 6918.
DOI: 10.1021/acs.joc.3c00216).
Haiyan Fu and Hua Chen of Sichuan University rearranged the pyridinium salt 5
to the pyrrole 6
(J. Org. Chem. 2023, 88, 2809.
DOI: 10.1021/acs.joc.2c02472).
Yu Yuan and Xiaodong Jia of
Yangzhou University oxidized the
pyrrolidine 7 to the 4-bromopyrrole 8
(Chem. Eur. J. 334905-81-6 web 2023, 29, e202203654.
DOI: 10.1002/chem.202203654).
Laurel L. Schafer of the University of British Columbia assembled the
pyridine 11 by
combining the alkyne 9 with the unsaturated aldehyde 10
(J. Org. Chem. 2023, 88, 1378.
DOI: 10.1021/acs.joc.2c02155).
Satoshi Ueno of the Tokyo University of Technology and Ryoichi Kuwano of Kyushu
University added the enamine 13 to the ketone 12 to give the pyridine 14
(Chem. Lett. 2023, 52, 148.
DOI: 10.1246/cl.220546).
Miroslav Soural of Palacky University rearranged the resin-bound sulfonamide 15 to an intermediate
that on spontaneous oxidation and release from the resin led to the pyridine 16
(J. Org. Chem. 2023, 88, 3228.
DOI: 10.1021/acs.joc.2c03025).
Sanghee Kim of Seoul National University prepared the
pyridinium salt 19 by combining the alkyne 17 with the aldehyde 18
(Chem. Eur. J. 2023, 29, e202300059.
DOI: 10.1002/chem.202300059).
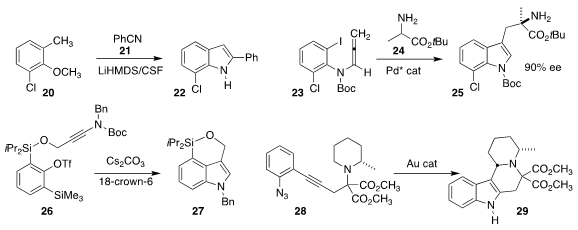
Jianyou Mao of Nanjin Tech University, Jinlin Zhang and Lai Chen of Hebei Agricultural University, and Patrick J. Walsh
of the University of Pennsylvania assembled the indole
22 by adding the anion derived from 20 to the nitrile 21
(J. Org. Chem. 2023, 88, 5147.
DOI: 10.1021/acs.joc.2c02128).
Qi-Xiang Guo of Southwest University achieved
substantial enantioselectivity in the assembly of the indole 25 by the coupling
of the allene 23 with the β-amino ester 24
(Org. Lett. 2023, 25, 3163.
DOI: 10.1021/acs.orglett.3c01119).
Kiyosei Takasu and Hiroshi Takikawa of Kyoto University showed that the benzyne derived
from 26 added to the pendant alkyne, leading to the indole 27
(Angew. Chem. Int. Ed. 2023, 62, e202300907.
DOI: 10.1002/anie.202300907).
Fabien Gagosz of the University of Ottawa achieved
high diastereoselectivity in the C-H insertion route that converted the azido
alkyne 28 to the indole 29
(Angew. Chem. Int. Ed. 2023, 62, e202212893.
DOI: 10.1002/anie.202212893).
Hiroaki Ohno, also of Kyoto University, reported related results
(Angew. Chem. Int. Ed. 2023, 62, e202213653.
DOI: 10.1002/anie.202213653).
Anibamine B (33), isolated from the neotropical tree Aniba sp., shows potential
activity vs. ovarian cancer. Jieping Zhu of the Ecole Polytechnique Fédérale de
Lausanne prepared the central pyridine 32 by the Cu-catalyzed addition of the
oxime acetate 30 to the alkyne 31
(Angew. Chem. Int. Ed. 2023, 63, e202303537.
DOI: 10.1002/anie.202303537).