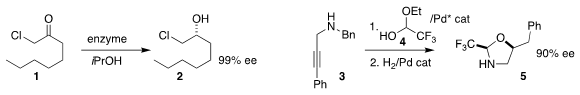
Vicente Gotor-Fernández and Iván Lavandera of the Universidad de Oviedo
optimized the enzymatic reduction of the
chloroketone 1 to the chlorohydrin
2, then telescoped that to begin with the 1-chloroalkyne (not illustrated)
(Org. Biomol. Chem. 2022, 20, 9650.
DOI: 10.1039/D2OB01953A).
Jerome Waser of the Ecole Polytechnique Fédérale de Lausanne used a Pd catalyst to combine the
propargyl amine 3 with the hemiacetal 4, then hydrogenated the product, leading to the
protected 1,2-aminoalcohol 5
(ACS Catal. 1-(2-N-Boc-aminoethyl)piperazine web 2022, 12, 7565.
DOI: 10.1021/acscatal.2c01809).
Robert Vianello and Maja Majerić Elenkov of the Ruder Boković Institute
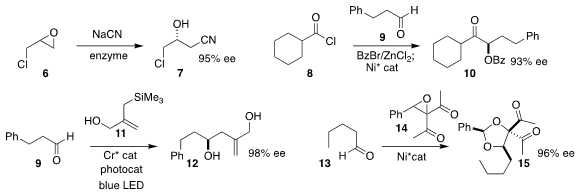
combined sodium cyanide with an enzyme to convert racemic epichlorohydrin 6 to the
β-hydroxy nitrile 7
(Adv. Synth. 2,4,5-Trichloroquinoline Chemscene Catal. 2022, 364, 2576.
DOI: 10.1002/adsc.202200342).
Qiaorong Han and Liang-An Chen of Nanjing Normal University assembled the
keto benzoate
10 by coupling the aldehyde 9 with the acid chloride 8
(J. PMID:23771862 Am. Chem. Soc. 2022, 144, 23019.
DOI: 10.1021/jacs.2c10072).
Frank Glorius of the Westfälische Wilhelms-Universität Münster used a
chromium catalyst and light to mediate the
preparation of the alcohol
12 by the addition of the allyl silane 11 to the aldehyde 9
(ACS Catal. 2022, 12, 12281.
DOI: 10.1021/acscatal.2c03960).
Hiroyuki Suga of Shinshu University constructed the protected diol 15 by adding
the epoxide 14 to the aldehyde 13
(Org. Lett. 2022, 24, 4739.
DOI: 10.1021/acs.orglett.2c01682).
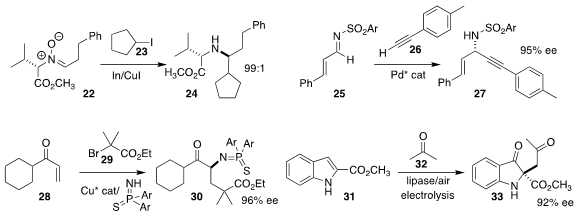
Johannes M. Wahl of Johannes Gutenberg-Universität Mainz used the reagent 17
to convert the cyclobutanone
16 to the lactam 18
(Org. Lett. 2022, 24, 6171.
DOI: 10.1021/acs.orglett.2c02361).
Enrique Gómez-Bengoa of the University of the Basque Country UPV/EHU and Rafael
Chinchilla of the University of Alicante used a diamine-derived
organocatalyst to
mediate the addition of the aldehyde 19 to the azodicarboxylate 20, leading to
the α-quaternary amine 21
(J. Org. Chem. 2022, 87, 14507.
DOI: 10.1021/acs.joc.2c01919).
Yongsheng Yang of Wuhan Textile University achieved high diastereoselectivity
in the reductive addition of the iodide 23 to the valine derivative 22, leading
to 24
(Tetrahedron 2022, 123, 132965.
DOI: 10.1016/j.tet.2022.132965).
René Peters of the Universität Stuttgart
used a Pd catalyst to mediate the addition of the alkyne 26 to the imine
25 to give the propargyl amine 27
(Angew. Chem. Int. Ed. 2022, 61, e202206835.
DOI: 10.1002/anie.202206835).
Yu-Feng Zhang, Zhong-Liang Li and Xin-Yuan Liu of the Southern University of
Science and Technology assembled the amine 30 by adding the α-bromoester
29 to the enone 28
(J. Am. Chem. Soc. 2022, 144, 18081.
DOI: 10.1021/jacs.2c08035).
Yan-Hong He and Zhi Guan of
Southwest University and Chu-Sheng Huang of Nanning Normal University used
electrolysis to prepare the diketone 33 by the oxidization of the
indole 31 in
the presence of acetone 32
(Angew. Chem. Int. Ed. 2022, 61, e202203666.
DOI: 10.1002/anie.202203666).
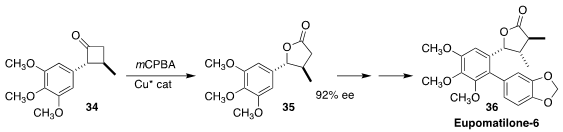
Eupomatilone-6 (36) was isolated from the Australian shrub Eupomatia bennettii.
En route to 36, Fu-Min Zhang of Wuyi University and Yong-Qiang Tu of Lanzhou
University prepared the lactone
35 by the enantioselective
Baeyer-Villiger
oxidation of the racemic cyclobutanone 34
(Chem. Sci. 2022, 13, 8429.
DOI: 10.1039/D2SC02079C).