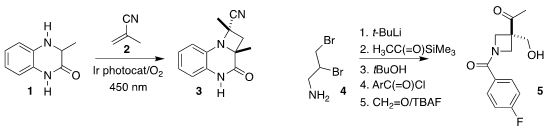
Malte Brasholz of the University of Rostock developed a protocol for the
photochemically-driven in situ oxidation of the quinoxalinone 1 followed by
[2+2] cycloaddition with methacrylonitrile 2 to give the
azetidine 3 with high
diastereocontrol
(Org. Lett. 2022, 24, 8041.
DOI: 10.1021/acs.orglett.2c03291).
Varinder K. Aggarwal of the University of Bristol assembled the azetidine 5 by a telescoped series of
transformations from the dibromo amine 4
(Angew. Formula of 16-Aminohexadecanoic acid Chem. Int. PMID:29844565 Ed. 2022, 61, e202114049.
DOI: 10.1002/anie.202214049).
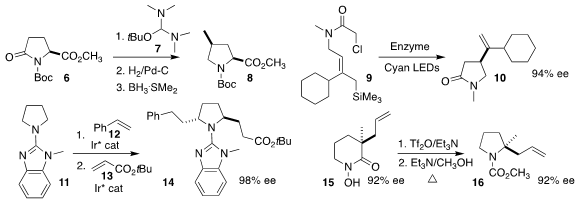
Marina Rubini of University College Dublin achieved high diastereoselectivity
in the conversion of the pyroglutamic acid derivative 6 to the protected
4-methyl proline 8, via initial condensation with Bredereck’s reagent (7)
(Org. Biomol. Chem. Buy7-Methyl[1,2,3]triazolo[1,5-a]pyridine 2022, 20, 6324.
DOI: 10.1039/D2OB01011A).
Todd K. Hyster of Princeton University effected the enantioselective enzymatic
cyclization of the chloroacetamide 9 to the
γ-lactam
10
(ACS Catal. 2022, 12, 9801.
DOI: 10.1021/acscatal.2c02294).
Takahiro Nishimura of Osaka
Metropolitan University added styrene (12) and t-butyl acrylate (13) sequentially
to the benzimidazolyl pyrrolidine 11 to give the
pyrrolidine 14 in high ee
(Org. Lett. 2022, 24, 6828.
DOI: 10.1021/acs.orglett.2c02733).
Brian M. Stoltz of Caltech developed conditions for
carbonyl extrusion to convert the N-hydroxylactam 15, prepared in high ee, to
the pyrrolidine 16
(Tetrahedron 2022, 123, 132940.
DOI: 10.1016/j.tet.2022.132940).
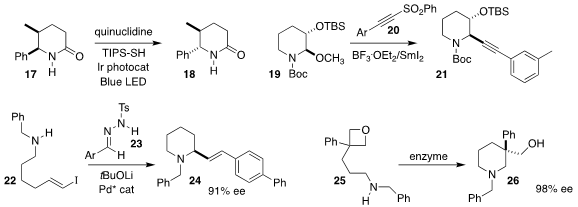
James M. Mayer and Jonathan A. Ellman of Yale University and Shuming Chen of
Oberlin College developed conditions for equilibrating the cis dialkyl δ-lactam
17 to the more stable trans dialkyl δ-lactam 17
(ACS Catal. 2022, 12, 7798.
DOI: 10.1021/acscatal.2c02232).
Bang-Guo Wei and Chang-Mei Si of Fudan University established a reductive
protocol for assembling the alkynyl
piperidine 21 by coupling the methoxy
piperidine 19 with the sulfonyl alkyne 20
(Chem. Commun. 2022, 58, 10841.
DOI: 10.1039/D2CC03984B).
Zhiming Li and Junliang Zhang, also of Fudan University, prepared the piperidine
24 by combining the alkenyl iodide 22 with the aryl diazomethane generated in
situ from the tosylhydrazone 23
(Chem. Sci. 2022, 13, 11150.
DOI: 10.1039/D2SC03999K).
Jun-An Ma of Tianjin University, Manfred T. Reetz of the Max-Planck-Institut für
Kohlenforschung and Zhoutong Sun of the Tianjin Institute of Industrial
Biotechnology designed an enzyme that cyclized the prochiral
oxetane 25 to the
piperidine 26 in high ee
(Nature Commun. 2022, 13, 7813.
DOI: 10.1038/s41467-022-35468-y).
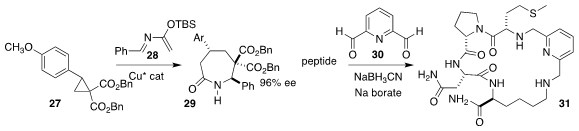
Stefano Nicolai and Jérôme Waser of the Ecole Polytechnique Fédérale de
Lausanne used a Cu catalyst to mediate the assembly of the lactam 29 by coupling
the racemic cyclopropane 27 with the imine 28
(Angew. Chem. Int. Ed. 2022, 61, e202209006.
DOI: 10.1002/anie.202209006).
Lara R. Malins of Australian National University established
reductive conditions for bridging the two free amines of an oligopeptide with
the dialdehyde 30 to give the cyclic peptide 31
(Org. Biomol. Chem. 2022, 20, 6250.
DOI: 10.1039/D2OB00782G).
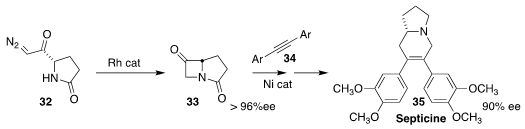
Septicine (35) was isolated from the Borneo fig Ficus septica. Janis Louie of
the University of Utah cyclized the pyroglutamic acid-derived diazo ketone 32 to
the α-lactam 33, and combined that with the alkyne 34, leading to
35
(J. Org. Chem. 2022, 87, 8871.
DOI: 10.1021/acs.joc.2c00365).