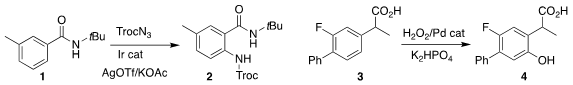Hongjian Lu of Nanjing University used an Ir catalyst to mediate the
regioselective ortho amination of the benzamide 1, leading to the protected
anthranilamide 2
(J. Org. 574007-66-2 structure Chem. 2022, 87, 13990.
DOI: 10.1021/acs.joc.2c01636).
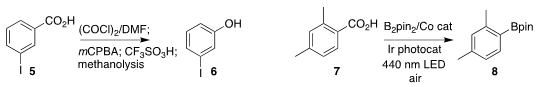
Jin-Quan Yu of Scripps/La
Jolla employed a Pd catalyst to direct the ortho
hydroxylation of the arylacetic
acid 3 to give the phenol 4
(J. Am. Chem. Soc. 2022, 144, 18109.
DOI: 10.1021/jacs.2c08332).
Wenbo H. Liu of Sun Yat-sen University oxidized the benzoic acid 5 to the phenol 6
(J. PMID:23290930 Am. Chem. Soc. 2022, 144, 15894.
DOI: 10.1021/jacs.2c07529).
Zhibo Liu of Peking University converted the benzoic acid 7 into the
aryl boronate 8
(Nature Commun. 2022, 13, 7112.
DOI: 10.1038/s41467-022-34833-1).
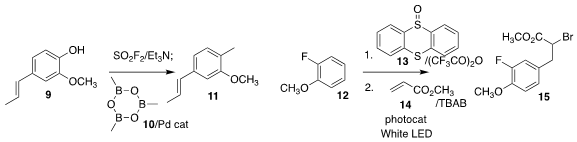
Chengrong Ding of the Zhejiang University of Technology activated the phenol
9 with SO2F2, then used a Pd catalyst to
couple the intermediate with
trimethylboroxine (10), leading to the methyl arene 11
(Org. Furo[3,2-c]pyridine site Biomol. Chem. 2022, 20, 7640.
DOI: 10.1039/D2OB01523D).
Tobias Ritter of the Max-Planck-Institut für Kohlenforschung further demonstrated the
versatility of aryl thianthrenium salts, activating the arene 12 with 13, then
coupling the intermediate with methyl acrylate 14 to give the α-bromo ester 15
(Angew. Chem. Int. Ed. 2022, 61, e202209882.
DOI: 10.1002/anie.202209882).
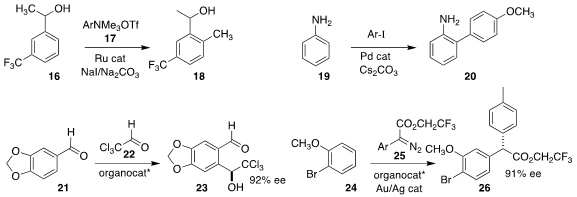
Igor Larrosa of the University of Manchester assembled the arene 18 by
effecting the regioselective ortho methylation of the benzylic alcohol 16, using
the ammonium salt 17 as the methyl donor
(JACS Au 2022, 2, 2529.
DOI: 10.1021/jacsau.2c00399).
Augustí Lledós of the Universitat Autònoma de Barcelona and Ana C. Albéniz of the Universidad
de Valladolid prepared the biphenyl 20
by the ortho arylation of the aniline 19
(ACS Catal. 2022, 12, 14527.
DOI: 10.1021/acscatal.2c05206).
Jan Otevrel and Pavel Bobal of Masaryk University
Brno used a Cinchona derived catalyst to mediate the enantioselective assembly of the
benzylic alcohol 23 by
the coupling of the benzaldehyde 21 with chloral 22
(Adv. Synth. Catal. 2022, 364, 2174.
DOI: 10.1002/adsc.202200180).
Lu Liu of East China Normal University
employed a BINOL-derived phosphoric acid to direct the Au/Ag-catalyzed coupling of the α-diazo ester
25 with the arene 24, leading to the aryl acetate 26 in high ee and with high regioselectivity
(Angew. Chem. Int. Ed. 2022, 61, e202208874.
DOI: 10.1002/anie.202208874).
Janakiram Vaitla of the Indian Institute of Technology Delhi combined the
sulfonium ylide 27 with ethyl acrylate 28 to give the
benzoate 29
(Org. Lett. 2022, 24, 8359.
DOI: 10.1021/acs.orglett.2c03388).
Zhi-Shi Ye of the Dalian University of Technology used a
phosphine catalyst to prepare the trisubstituted benzene 32 by the coupling of
the cyclopropyl acetylene 30 with the alkyne 31
(Org. Lett. 2022, 24, 6489.
DOI: 10.1021/acs.orglett.2c02201).
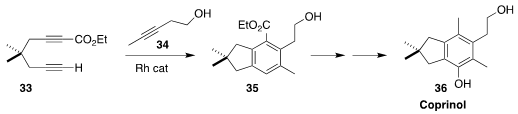
Coprinol (36) is an illudalane sesquiterpene isolated from the edible mushroom
Coprinopsis cinerea. Gregory B. Dudley of the West Virginia University assembled
the bicyclic core 35 of 36 by the Rh-catalyzed
aromatizing combination of the
alkyne 34 with the diyne 33
(J. Org. Chem. 2022, 87, 14909.
DOI: 10.1021/acs.joc.2c01741).