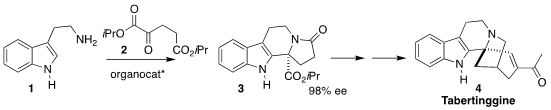
Tabertinggine (4) was isolated from the Mayasian shrub
Tabernaemontana corymbosa, the dwarf pinwheel. Xuegong She of Lanzhou
University assembled the key tetracyclic intermediate 3 by the
BINOL-derived phosphoric acid mediated Pictet-Spenger combination of tryptamine
(1) with the α-keto diester 2
(Eur. J. Org. PMID:35227773 Chem. 2022, e202200088.
DOI: 10.1002/ejoc.202200088). Formula of 4-Bromo-2-methyl-1,3-thiazole
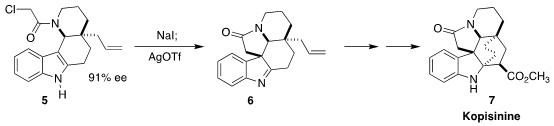
Kopisinine (7), isolated from the Southeast Asian tree Kopsia longiflora, shows
strong antitussive properties. En route to 7, Eelco Ruijter of the Vrije Universiteit Amsterdam
effected the cyclization of the chloroacetamide 5 to the pentacyclic 6
(Angew. Chem. Int. Ed. 2022, e202210592.
DOI: 10.1002/anie.202210592). 109705-14-8 Chemscene
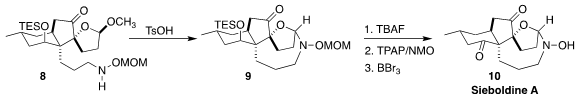
Sieboldine A (10), isolated from the club moss Lycopodium sieboldii, is an
inhibitor of acetylcholine esterase. Zhu-Jun Yao of Nanjing University prepared
the amine 9 by acid-mediated cyclization of the hemiacetal 8
(Org. Lett. 2022, 24, 7517.
DOI: 10.1021/acs.orglett.2c02737).
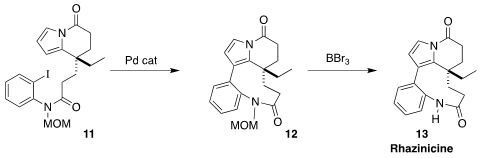
Rhazinicine (13), isolated from Kopsia dasyrachis, a tree of Sabah, Borneo,
showed selective cytotoxicity in vitro. Following the precedent of Trauner
(![]()
2006, March 27), Luis D. Miranda of the Universidad
Nacional Autónoma de México assembled the macrocyclic lactam of 13 by the
Pd-mediated cyclization of 11 to 12
(Org. Lett. 2022, 24, 8093.
DOI: 10.1021/acs.orglett.2c02446).
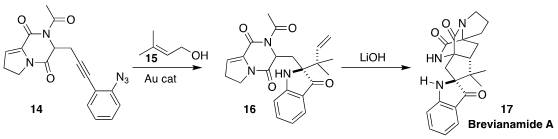
Brevianamide (17), isolated from the fungus Penicillium brevicompactum,
demonstrated potent antifeedant activity. Fabien Gagosz of the University of
Ottawa assembled the amine 16 by Au-catalysed combination of the alkyne azide 14
with prenyl alcohol 16, and showed that on hydrolysis it spontaneously cyclized to 17
(Org. Lett. 2022, 24, 7200.
DOI: 10.1021/acs.orglett.2c02971).
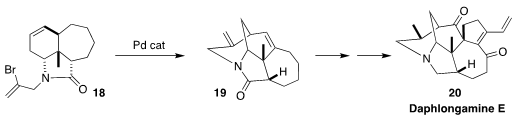
Daphlongamine E (20) was isolated from the Chinese evergreen tree Daphniphyllum
longeracemosum. In a continuation of their studies of the Daphniphyllum
alkaloids, Jing Xu of the Southern University of Science and Technology described
the Heck cyclization of the alkenyl bromide 18 to the tetracyclic lactam
19
(J. Am. Chem. Soc. 2022, 144, 16042,
DOI: 10.1021/jacs.2c05957;
Org. Lett. 2022, 24, 7416,
DOI: 10.1021/acs.orglett.2c02988).