Triptonide (3), isolated from the Chinese herb Tripterygium Wilfordii
Hook F, displays reversible male contraceptive effects in both mice and monkeys.
A key step in the synthesis of 3 by Tuoping Luo of Peking University was
the cyclization of the diene 1 to the aldehyde 2
(J. Am. Chem. Soc. Price of 917397-92-3 2022, 144, 2292.
DOI: 10.1021/jacs.1c12525). PMID:23800738 2135443-03-5 site
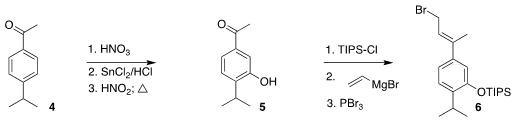
The convergent assembly of the diene 1 began with commercial 4.
Nitration followed by
reduction and
diazonium salt formation led to the phenol
5. Protection, addition of vinyl magnesium bromide and brominative
rearrangement then completed the preparation of the
allylic bromide 6.
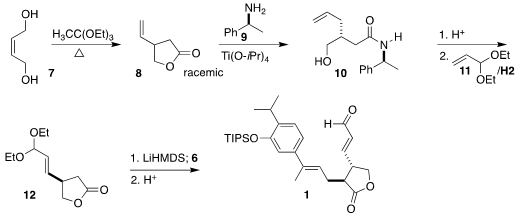
The construction of the lactone began with the inexpensive diol 7.
Johnson-Claisen rearrangement led to racemic 8. Opening with 9 led
to the expected 1:1 mixture of diasteromers, from which the desired 10 could be crystallized. Treatment with acid reformed the lactone, that
on cross metathesis with the acetal 11 gave the lactone 12.
Alkylation with 6 followed by acid hydrolysis then completed the
synthesis of 1.
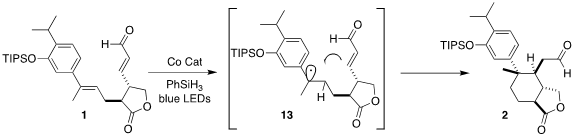
The authors envisioned a free radical cyclization of 1 initiated by hydrogen
atom transfer to the styrenic alkene, to give 13. In the event, it took
considerable experimentation to define conditions for efficient cyclization to
2.
Deprotection of the phenol of 2 followed by Ti-mediated cyclization
delivered the secondary benzylic alcohol with high diastereocontrol. Followed
previous syntheses in this series by others,
peridoate oxidation then gave the
epoxide 14, that could be sequentially
epoxidized to 15.
Selenation and elimination completed the synthesis of triptonide 3.
The H-atom transfer conditions developed by the authors in the course of this
synthesis will have many other applications. They briefly explored some of that
scope, and report their findings.