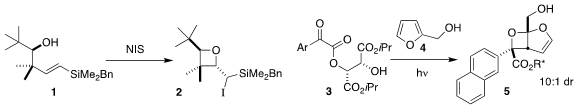
Mark G. 259214-55-6 uses McLaughlin of the University of Lancaster found that under alkaline
conditions, the alcohol 1 could be cyclized to the iodo oxetane 2
(Chem. Commun. 2022, 58, 8376.
DOI: 10.1039/D2CC03339A).
Yutaka Ukaji of Kanazawa University observed high asymmetric
induction in the [2+2] photocycloaddition of the α-keto ester 3 with furfuryl
alcohol 4 to give the
oxetane 5
(Chem. Lett. 2022, 51, 1143.
DOI: 10.1246/cl.220437). PMID:24282960
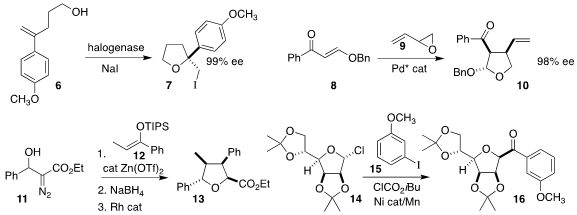
Jared C. Lewis of Indiana University showed that a flavin-dependent halogenase
could cyclize the alcohol 6 to the
tetrahydrofuran
7 in high ee
(Angew. Chem. Int. Ed. n-Octyl β-D-glucopyranoside manufacturer 2022, 61, e202214610.
DOI: 10.1002/anie.202214610).
Yong Jian Zhang of Shanghai Jiao Tong University combined the enone 8
with the racemic epoxide 9 to give the tetrahydrofuran 10 in high ee
(Org. Lett. 2022, 24, 6716.
DOI: 10.1021/acs.orglett.2c02437).
Matthias Brewer of the University of
Vermont observed high diastereoselectivity in the assembly of the tetrahydrofuran
13 by coupling the silyl enol ether 12 wih the α-diazo ester 11
(Tetrahedron Lett. 2022, 109, 154137.
DOI: 10.1016/j.tetlet.2022.154137).
Shi-Jun Li of Zhengzhou University, Yu
Lan of Chongqing University, and Quanquan Wang and Ming Joo Koh of the National
University of Singapore devised the three-component coupling of the glycosyl
chloride 14, the iodoarene 15 and isobutyl chloroformate to prepare the C-acyl
glycoside 16
(Angew. Chem. Int. Ed. 2022, 62, e202211043.
DOI: 10.1002/anie.202211043).
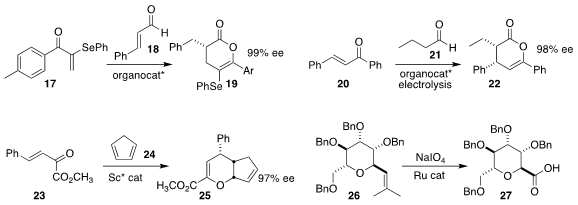
Bhoopendra Tiwari of the Centre of Biomedical Research used a triazolium NHC catalyst
to mediate the coupling of the α-selenyl enone 17 with cinnamaldehyde 18
to give the enol lactone 19
(Adv. Synth. Catal. 2022, 364, 4031.
DOI: 10.1002/adsc.202201036).
Tingshun Zhu of Sun Yat-sen University used the same triazolium NHC catalyst to prepared
the enol lactone 22 by combining the chalcone 20 with butanal 21
(Nature Commun. 2022, 13, 3827.
DOI: 10.1038/s41467-022-31453-7).
Zheng-Hang Qi of the University of Science and Technology of
China and Yong Wang and Xing-Wang Wang of Soochow University devised the hetero
Diels-Alder addition of the α-keto ester 23 to cyclopentadiene
24, leading to
the bicyclic 25
(Adv. Synth. Catal. 2022, 364, 4347.
DOI: 10.1002/adsc.202200875).
Pei-Xin Rui and Xiang-Guo
Hu of Jiangxi Normal University showed that the alkenyl glycoside 26 could be
cleanly oxidized to the glycosyl carboxylic acid 27
(Org. Biomol. Chem. 2022,
20, 5452.
DOI: 10.1039/D2OB00896C).
Ai-Jun Ma of Wuyi University effected the oxidative
ring expansion of the
cyclopentanone
28 to the macrolactone 29
(Adv. Synth. Catal. 2022, 364, 2152.
DOI: 10.1002/adsc.202200192).
Hyoungsu Kim of Ajou University cyclized the tartrate-derived bis-tosylate 30 to
the bicyclic amide 31 with high diastereocontrol
(Org. Lett. 2022, 24, 8780.
DOI: 10.1021/acs.orglett.2c03494).
Miharimycin B (34), isolated from Streptomyces miharaensis, inhibited the
growth of the plant pathogenic fungus Pyricularia oryzae at 10-20 ppm. Jian Liu
and Xiaolei Wang of Lanzhou University assembled the trans 6/5 bicyclic core of
34 by the MsCl-mediated cyclization of the carbohydrate-derived polyol 32 to 33
(Angew. Chem. Int. Ed. 2022, 61, e202204907.
DOI: 10.1002/anie.202204907).