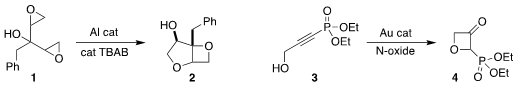
Arjan W. Kleij of ICIQ optimized an aluminum catalyst for the
conversion of the bis epoxide 1 to the
oxetane 2
(ACS Catal. PMID:23399686 2022, 12, 5464.
DOI: 10.1021/acscatal.2c00925).
A. Stephen K. Formula of Fmoc-Ala-OH Hashmi of Heidelberg University used a gold catalyst to rearrange
the alkyne 3 to the
oxetanone 4,
that participated readily in
Horner-Wadsworth-Emmons reactions
(Org. Chem. Fmoc-Gly-OH custom synthesis Front. 2022, 9, 117.
DOI: 10.1039/D1QO01214B).
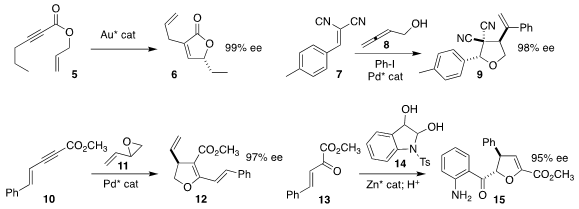
Ting Li of Nanyang Normal University, Jun Zhu of Xiamen University and Liming
Zhang of the University of California, Santa Barbara achieved high enantioselectivity
in the rearrangment of the allyl alkynoate 5 to the
lactone 6
(Org. Lett. 2022, 24, 4427.
DOI: 10.1021/acs.orglett.2c01652).
Qun Li and Yong Jian Zhang of Shanghai Jiao Tong University also observed high ee
in the construction of the
tetrahydrofuran
9 by the three-component coupling
of the benzylidene malononitrile 7, the allene 8, and iodobenzene
(Org. Lett. 2022, 24, 2081.
DOI: 10.1021/acs.orglett.2c00142).
Xue-Long Hou of the Shanghai Institute of Organic Chemistry assembled the
dihydrofuran
12 by the combination of the enyne 10 with butadiene monoepoxide 11
(Org. Lett. 2022, 24, 1561.
DOI: 10.1021/acs.orglett.2c00253).
Yuan-Zhao Hua, Shi-Kun Jia and Min-Can Wang of Zhengzhou University used a
dinuclear zinc catalyst to mediate the coupling of the enone 13 with the diol
14, leading to the dihydrofuran 15
(Org. Lett. 2022, 24, 3909.
DOI: 10.1021/acs.orglett.2c00913).
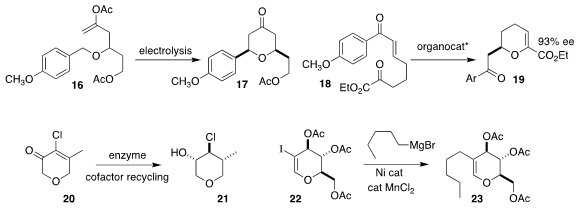
Paul E. Floreancig of the University of Pittsburgh used electrolysis to drive
the cyclization of the enol acetate 16 to the tetrahydropyranone 17
(Chem. Eur. J. 2022, 28, e202200335.
DOI: 10.1002/chem.202200335).
Keisuke Asano of Hokkaido University and Seijiro Matsubara of Kyoto
University employed a Cinchona-derived catalyst to mediate the cyclization of
the enone 18 to the
dihydropyran 19
(Tetrahedron 2021, 97, 132381.
DOI: 10.1016/j.tet.2021.132381).
Francesco G. Gatti of the Politecnico di Milano showed that a combination of two enzymes
reduced the enone 20 to the
tetrahydropyran 21
(J. Org. Chem. 2022, 87, 6499.
DOI: 10.1021/acs.joc.2c00427).
Janine Cossy of ESPCI Paris showed that the 2-iodoglycal 22 could be coupled
with a range of Grignard reagents, as illustrated by the formation of the dihydropyran 23
(Chem. Eur. J. 2022, 28, e202104311.
DOI: 10.1002/chem.202104311).
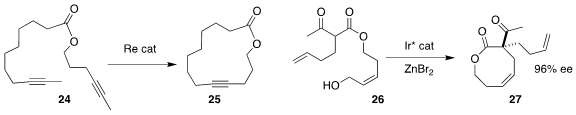
Ian D. Williams and Guochen Jia of the Hong Kong University of Science and
Technology developed a Re catalyst for the ring-closing metathesis of the diyne
24, leading to the macrolactone 25
(J. Am. Chem. Soc. 2022, 144, 6349.
DOI: 10.1021/jacs.2c00368).
Shu-Li You, also of the Shanghai Institute of Organic Chemistry, achieved high
enantiomeric excess in the Ir-mediated cyclization of the Z-allylic alcohol to
the macrolactone 27
(J. Am. Chem. Soc. 2022, 144, 4770.
DOI: 10.1021/jacs.2c01103).
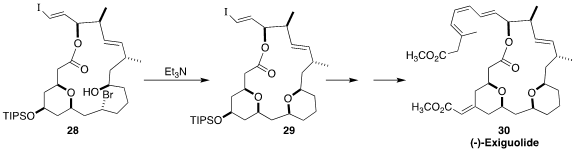
Exiguolide (30), isolated from the rare marine sponge Geodia exigua, showed
potent anti-proliferative activity against human non-small cell lung cancer cell
lines. In the course of a synthesis of 30, Haruhiko Fuwa of Chuo University
cyclized the bromo alcohol 28 to the tetrahydropyran 29
(Angew. Chem. Int. Ed. 2022, 61, e202202549.
DOI: 10.1002/anie.202202549).