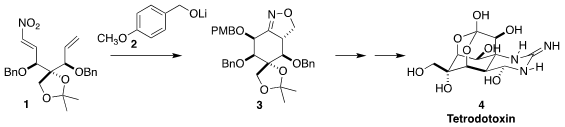
Tetrodotoxin (4), the lethal principle of pufferfish (and some
salamanders!) is a highly-substituted cyclohexane. Buy2179072-33-2 Dirk Trauner of the
University of Pennsylvania assembled the intermediate cyclohexane 3 via
an intramolecular
dipolar
cyclization initiated by the conjugate addition of the alkoxide 2 to
the nitroalkene 1
(Science 2022, 377, 411
DOI: 10.1126/science.abn0571). PMID:23880095 Dde-Dap(Fmoc)-OH Order
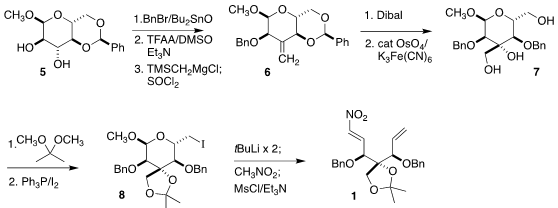
The synthesis began with the glucose derivative 5.
Benzylation
followed by oxidation and
methylenation led
to the alkene 6. Selective reduction followed by
catalytic osmylation
gave the diol 7. Formation of the
acetonide followed by
an Appel reaction
delivered the iodide 8, that was reduced with tBuLi. Nitromethane
was added to the resulting aldehyde to give, after dehydration, the nitroalkene
1.
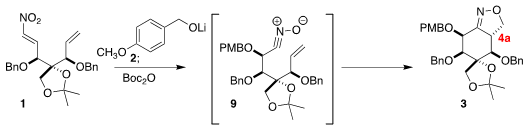
Conjugate addition
of the alkoxide 2 to 1 proceeded with high diastereoselectivity,
leading to the intermediate nitronate, that on exposure to Boc2O was
converted into the nitrile oxide 9. The subsequent dipolar cycloaddition
proceeded with high diastereoselectivity, but to give 3, having the wrong
relative configuration at 4a (tetrodotoxin numbering) for the natural product.
In fact, this was advantageous.
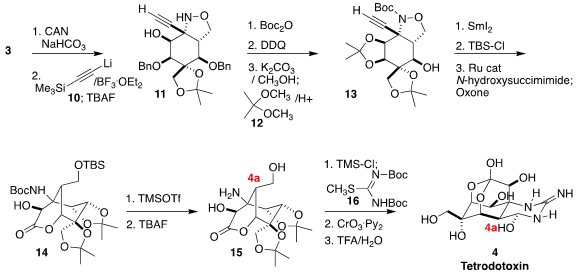
The importance of the axial substituent at 4a became apparent in the next
step. Selective deprotection of 3 led to the free alcohol. The addition
of the alkynyl anion 10 then proceeded with high diastereoselectivity, to
give, after deprotection, the α-quaternary amine 11. Protection with
12 led to
the acetonide 13, that was reduced and selectively protected, then
cyclized and oxidized to the α-hydroxy lactone 14. Deprotection delivered the amino diol
15,
that was protected, condensed with the reagent 16 to form the
guanidine, then
oxidized with Collins reagent to give an intermediate aldehyde that epimerized
at 4a to the more stable equatorial configuration in the course of the
cyclization to tetrodotoxin (4).
It is instructive to compare this synthesis to the complementary approaches
described by Minoru Isobe (Highlights May 2, 2005) and Tohru Fukuyama
(Highlights May 7, 2018).