Gedunin (3), isolated from the Indian neem tree Azadirachta indica, modulates the activity of Hsp90. PMID:23907051 En route to
3,
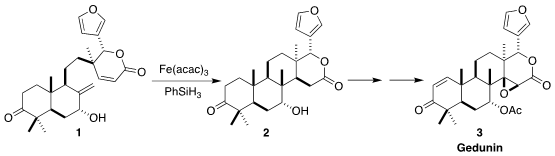
Hans Renata of Rice University devised the diastereoselective H-atom transfer cyclization of
1 to 2
(J. Am. Chem. Price of 2-(3-Fluoro-2-methoxyphenyl)acetic acid Soc. 2022, 144, 19238.
DOI: 10.1021/jacs.2c09048).
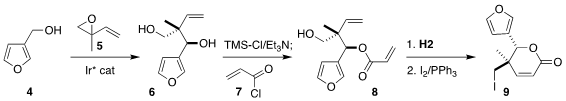
The convergent assembly of the triene 1 began with furfuryl alcohol 4 and racemic isoprene monoepoxide
5. Following the Krische protocol, coupling of the
two led to the diol 6 with high diastereo- and enantioselectivity. The derived
monoacrylate 8 was cyclized, then carried on to the iodide 9. Formula of Non-8-yn-1-ol
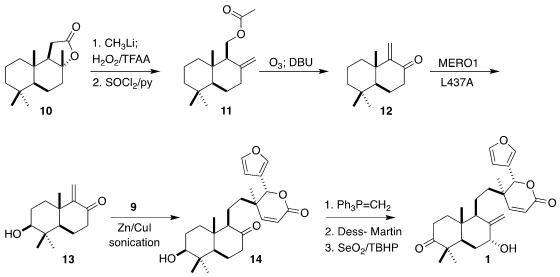
The inertness of the A ring of sclareolide 10 allowed ready conversion, via
methyl ketone formation,
Baeyer-Villiger oxidation and dehydration, to the
alkene 11. Ozonolysis followed by β-elimination delivered the
enone 12, that was
oxidized by a cytochrome P450 variant to install the equatorial secondary
hydroxyl on the A ring. Reductive conjugate addition of 9 to 13 gave the ketone
14, that was carried on to 1.
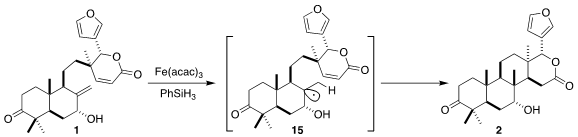
The cyclization of 1 to 2 proceeded by reductive H-atom transfer, presumably via
the intermediate free radical 15.
Conversion of the ketone 2 to the corresponding bis silyl enol ether
necessitated concomitant silylation of the secondary alcohol. After Pd-mediated
oxidation, desilylation followed by acetylation led to the diene 16. Diastereoselective
epoxidation completed the synthesis of gedunin (3).
In the words of the authors regarding the selective cytochrome P450 variants
used in this study, "although virtual docking was used to rationalize empirical
observations after the fact and in related studies, its general agreement with
experimental results points to the possibility of using virtual prescreening of
substrate candidates in future chemoenzymatic route development."