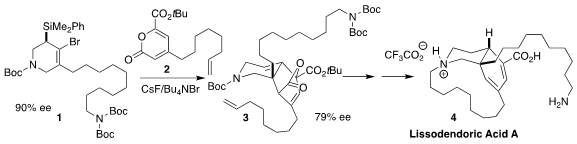
Lissodendoric acid A (4), isolated from the marine sponge
Lissodendoryx florida, reduced reactive oxygen species (ROS) in a
Parkinson’s disease model. Fmoc-β-HoGlu(OtBu)-OH Chemscene Neil K. Garg of UCLA devised an approach to 4
based on the generation of the cyclic
allene from 1, and in situ
cycloaddition to the 2-pyrone 2 to give 3
(Science 2023, 379, 261.
DOI: 10.1126/science.ade0032).
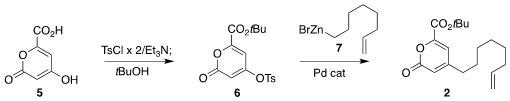
The 2-pyrone 2 was prepared from the commercial acid 5. PMID:23916866 5-Iodopyrimidine Formula Bis tosylation
followed by quenching with t-butanol led to the ester 6. Coupling with the
organozinc 7 then completed the preparation of 2.
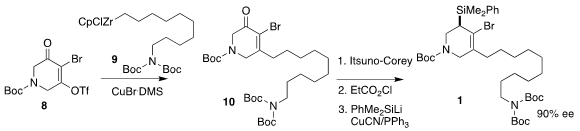
The bromide 1 was assembled from the commercial triflate 8. Coupling with the
organozirconium reagent 9 gave 10. The absolute configuration was then set by
enantioselective reduction to the allylic alcohol. Coupling of the derived ester
with the PhMe2SiLi led to 1 in 90% ee.
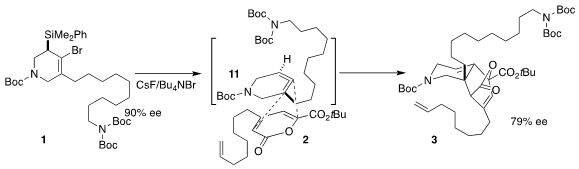
The cyclic allene 11 has two alkenes, and each has two faces. Further, the
barrier to racemization of the allene is estimated to be only ~ 14 kcal/mol.
Nevertheless, the cycloaddition proceeded predominantly across just one face of
one of the alkenes, and with substantial maintenance of the enantiomeric excess.
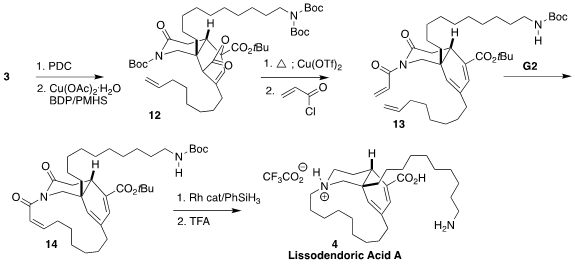
To complete the synthesis, it was necessary to selectively reduce one of the
two alkenes. To this end, the
cyclic amine was oxidized to the
unsaturated
lactam, then reduced in a conjugate sense with "CuH", leading to
12. Extrusion
of CO2 followed by removal of two of the three
Boc groups and acylation with
acryloyl chloride gave 13.
Ring-closing metathesis led to
14. Reduction followed
by removal of the t-butyl ester completed the synthesis of lissodendoric acid A
(4).