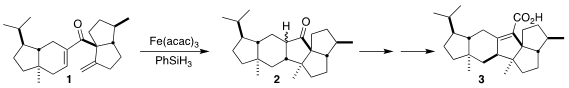
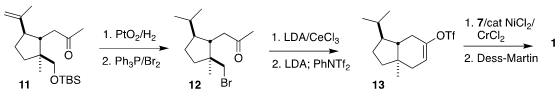Retigeranic acid (3), isolated from the Western Himalayas lichen
Lobaria retigera, presents a variety of intriguing architectural challenges.
Xiaoming Chen and Shao-Hua Wang of Lanzhou University devised a concise route to
3 based on the H-atom transfer cyclization of the diene 1 to the
ketone 2
(J. 2,3-Dibromopropene structure Am. Chem. PMID:24118276 Soc. 2023, 145, 13549.
DOI: 10.1021/jacs.3c04850).
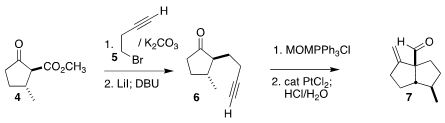
The starting material for the convergent
assembly of 1 was the β-keto ester 4, readily prepared from pulegone. Alkylation
with the bromide 5 followed by demethoxycarbonylation led to the cyclopentanone
6. One carbon homologation followed by modified
Conia cyclization completed the
synthesis of the aldehyde 7. 2-Bromo-5-methylthiazole-4-carbonitrile site
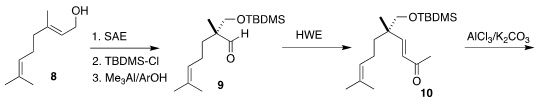
The other cyclic quaternary center of 1 was constructed by
Sharpless
asymmetric epoxidation of geraniol 8 followed by protection and Lewis
acid-mediated pinacol rearrangement to give
9. The corresponding enone 10 was
cyclized to the ketone 11. Following the House precedent established decades ago,
intramolecular alkylation of the derived bromide proceeded smoothly, leading to
the triflate 13. Coupling with the aldehyde 7 completed the assembly of the
ketone 1.
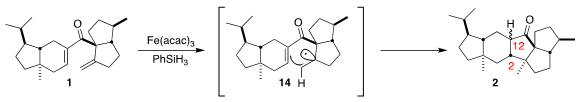
The conversion of 1 to 2 presumably proceeded by H atom addition to the exo
methylene, leading to the free radical 14. Further cyclization then gave
2.
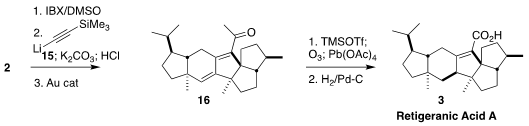
The ketone 2 had the wrong relative configuration at C-2, and was a mixture
of epimers at C-12. Addition of the lithium acetylide 15 followed by dehydration
and Au-catalyzed conversion of the alkyne to the ketone delivered the diene 16.
Oxidative cleavage to the corresponding carboxylic acid followed by selective
hydrogenation completed the synthesis of retigeranic acid (3).
Five weeks earlier, Hanfeng Ding of Zhejiang University published an
alternative route to retigeranic acid (3)
(J. Am. Chem. Soc. 2023, 145, 11927.
DOI: 10.1021/jacs.3c03178).
It is instructive to compare and contrast these two approaches.