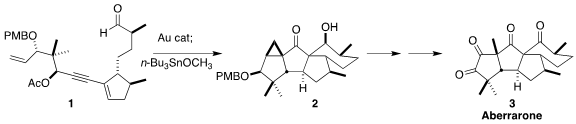
The tetraketone aberrarone (3) was isolated from the feathery Caribbean gorgonian
Pseudopterogorgia elisabethae.
En route to 3, Erick M. Carreira of ETH Zürich devised the one-pot, two-step assembly of the pentacyclic
aldol product
2 from the substituted
cyclopentene
1
(J. PMID:23613863 Exatecan Intermediate 2 uses Am. Chem Soc. 2022, 144, 15475.
DOI: 10.1021/jacs.2c07150).
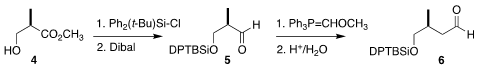
The cyclopentene 1 was assembled from three components, Roche ester
4, pantolactone (7), and 3-methylcyclopentenone (11). The
Roche ester 4 was
silylated, then reduced with
Dibal to the
aldehyde 5. Price of 874-20-4 Methoxymethylenation followed by hydrolysis converted
5 into the aldehyde 6.
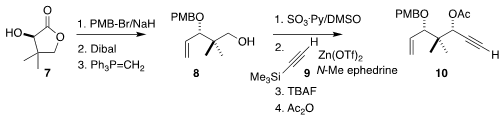
Pantolactone (7) was protected, then reduced with Dibal and carried on
to the alkene 8. Oxidation followed by the addition of the alkyne 9,
desilylation and
acetylation
completed the assembly of the alkyne 10.
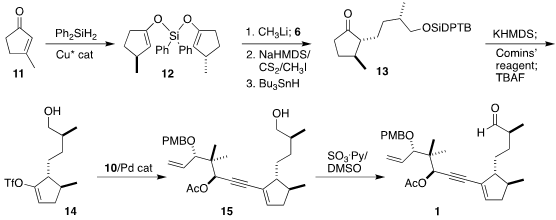
Following the Buchwald protocol,
3-methylcyclopentenone (11) was reduced to the bis-silyl enol ether 12.
Generation of the enolate followed by the addition of the aldehyde 6 led
to the alcohol as an inconsequential mixture of diastereomers, that were
converted into their respective xanthates and reduced to the ketone 13.
Conversion to the enol triflate 14 followed by coupling with the alkyne
10 led to 15, that was oxidized to the aldehyde 1.
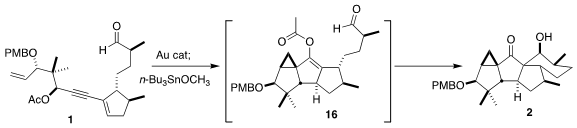
The initial product from the gold-catalyzed rearrangement of 1 was the
enol acetate 16. On combination with Bu3SnOCH3,
this was converted to the tin enolate, that added to the aldehyde, leading to
the pentacyclic 2.
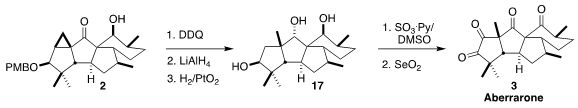
Hydrogenation of the deprotected ketone led to cleavage of the wrong
cyclopropane
bond, so after deprotection the ketone was reduced to the
alcohol.
Hydrogenolysis then delivered the triol 17. Oxidation to the triketone
followed by further oxidation with
selenium dioxide
completed the preparation of aberrarone (3).