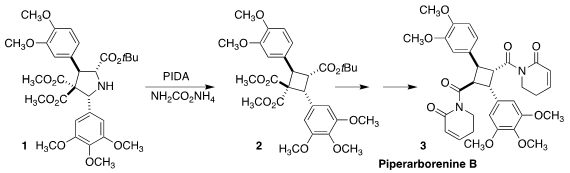
Piperarborenine B (3), isolated from the stem of Piper arborescens,
shows significant and selective antineoplastic activity. 2-(4,4-Difluorocyclohexyl)acetic acid site Andrey P. Antonchick of
Nottingham Trent University constructed the
cyclobutane core of 3 by
oxidative nitrogen extrusion from the pyrrolidine 1 to give 2
(J. Am. Chem. Soc. 2021, 143, 18864.
DOI: 10.1021/jacs.1c10175).
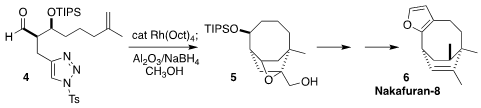
Nakafuran-8 (6), isolated from the marine sponge Dysidea fragilis
and from its prey, the nudibranchs Hypselodoris godeffroyana and
Chromodoris maridadilus, is an inhibitor of human tyrosine phosphatase 1B. 2169908-22-7 In stock
K. PMID:24078122 N. Houk of UCLA and Chuang-Chuang Li of the Southern University of Science
and Technology established the bicyclic core 5 of 6 by the [3+2]
intramolecular
dipolar cyclization of the N-sulfonyltriazole 4,
followed by hydrolysis and reduction
(Nature Commun. 2021, 12, 5239.
DOI: 10.1038/s41467-021-25513-7).
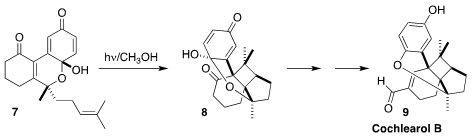
Cochlearol B (9) was isolated from the fruiting body of the fungus
Ganoderma cochlear, used in traditional Chinese medicine. En route to 9,
Kazuyuki Sugita of Hoshi University effected the intramolecular [2+2]
photocycloaddition of the
cyclohexenone 7 to 8
(Angew. Chem. Int. Ed. 2021, 60, 24484.
DOI: 10.1002/anie.202110556).
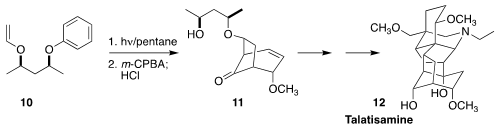
Talatisamine (12), a delphinine alkaloid extracted from the flowering
shrub Aconitum talassicum of Central Asia, is a K+ channel
blocker with hypotensive and antiarrhythmic activities. Sarah E. Reisman of
Caltech set the stage for the synthesis of 12 by the photocyclization of
the phenyl ether 10, followed by
epoxidation and rearrangement, to give
11
(ACS Cent. Sci. 2021, 7, 1311.
DOI: 10.1021/acscentsci.1c00540).
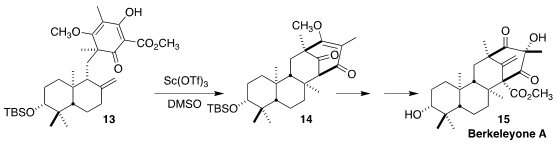
Berkeleyone A (15), isolated from a strain of the fungus
Penicillium rubrum growing in the Berkeley Pit, was found to have potent
anti-inflammatory activity. Houhua Li of Peking University constructed the
intermediate diketone 14 by Krapcho demethoxycarbonylation of 13
and subsequent cyclization
(Angew. Chem. Int. Ed. 2021, 60, 14869.
DOI: 10.1002/anie.202104014).
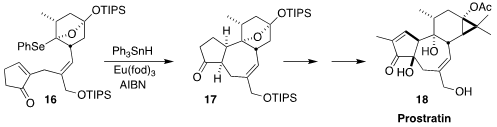
Prostratin (18), isolated from the bark of the mamala tree of Samoa,
Homalanthus nutans, strongly activates HIV replication in latently
infected cells. In the course of a synthesis of 18, Masayuki Inoue of the
University of Tokyo achieved high diastereoselectivity in the free radical
cyclization of the
cyclopentenone 16 to the tetracyclic 17
(J. Am. Chem. Soc. 2021, 143, 12387.
DOI: 10.1021/jacs.1c06450).