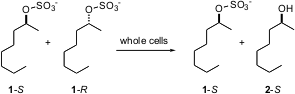High ee halogenated secondary centers and, via activation, oxygenated secondary centers are requisite intermediates for the preparation of enantiomerically-pure natural products and pharmaceuticals. Several methods have recently been reported for the conversion of achiral or prochiral starting materials into high ee intermediates.
A limitation on resolution is that the desired enantiomer is only half of the racemic starting material. Kurt Faber of the University of Graz has reported (Org. 457613-78-4 site Lett. 2004, 6, 5009. DOI: 10.1021/ol0477778)a clever solution to this problem. PMID:23771862 On exposure of the sulfate 1 of a secondary alcohol to aerobically grown whole cells of Sulfolobus acidocaldarius DSM 639, one enantiomer of the sulfate was smoothly converted into the other enantiomer of the starting alcohol. The enzyme consumed the more reactive enantiomer > 200 times more rapidly than the less reactive enantiomer. For the last bit of conversion, the ee of the product alcohol will of course fall. 2246363-82-4 Chemscene One solution to this would be to run the reaction near 50% conversion, then hydrolyze the mixture to give high ee product alcohol 2. Exposure of the mixture to a lipase that selectively acetylated the minor enantiomer would then polish the ee of 2.
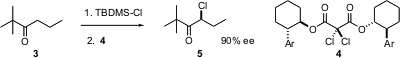
The ketone 3 is prochiral. Hisashi Yamamoto of the University of Chicago has shown (J. Am. Chem. Soc. 2004, 126, 15038. DOI: 10.1021/ja0454485)that conversion of a ketone such as 3 to its silyl enol ether followed by exposure to the chiral chlorinating agent 4 gives the chloro ketone 5 in high ee. The agent 4 is easily regenerated. Chloro ketones such as 5 can be reduced to the chloro alcohol with high diastereoselectivity.
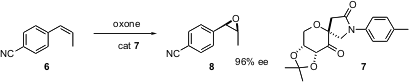
The epoxidation procedure developed by Yian Shi of Colorado State University has become one of the workhorses of enantioselective synthesis. That work has been based around trans and trisubstituted alkenes. Professor Shi has now developed (Tetrahedron Lett. 2004, 45, 8115.DOI: 10.1016/j.tetlet.2004.08.124)an efficient protocol for the enantioselective epoxidation of aryl-substituted cis alkenes such as 6.
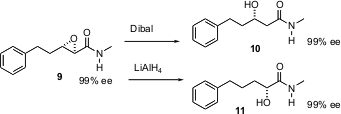
Masakatsu Shibasaki of the University of Tokyo has developed effective procedures for the epoxidation of α,β-unsaturated amides with high ee. He has now reported (Angew. Chem. Int. Ed. 2004, 43, 317. DOI: 10.1002/anie.200352431)reagents for the selective reduction of the epoxy amide 9 to either the β-hydroxy amide 10 or the α-hydroxy amide 11.
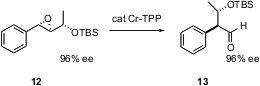
High ee epoxy alcohols such as 12 are prepared by Sharpless asymmetric epoxidation. Kohji Suda of Meiji Pharmaceutical University, Tokyo, has described (J. Am. Chem. Soc. 2004, 126, 9554.DOI: 10.1021/ja047104k)the optimization of Cr TPP as a catalyst for the rearrrangement of the epoxides such as 12 to the corresponding aldehyde. The reaction proceeds without loss of ee.