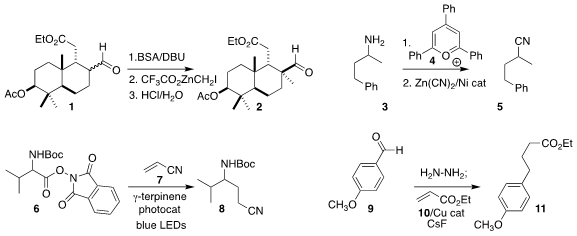
Following the Wenkert approach, Jun Huang and Zhen Yang of Peking University prepared the
alkylated aldehyde 2 by conversion of the aldehyde 1 to the silyl enol ether, followed by
cyclopropanation and hydrolysis
(J. Am. Chem. Soc. 2021, 143, 18287.
DOI: 10.1021/jacs.1c08880).
Mary P. Watson of the University of Delaware showed that the
pyridinium salt prepared by coupling the amine 3 with the commercial pyrilium
salt 4 could be coupled with Zn(CN)2
to give the nitrile 5
(Org. Lett. 3-Bromo-1,1-difluorocyclobutane web 2021, 23, 6242.
DOI: 10.1021/acs.orglett.1c01959).
Paolo Melchiorre of ICIQ used photoactivation to add the amino acid-derived acyloxyphthalimide
6 in a conjugate sense to acrylonitrile 7, leading to the nitrile 8
(J. Am. Chem. PMID:23912708 Soc. 2021, 143, 12304.
DOI: 10.1021/jacs.1c05607).
Chao-Jun Li of McGill University assembled the ester 11 by Cu-mediated conjugate
addition of the hydrazone of the aldehyde 9 to ethyl acrylate 10
(J. Org. Chem. Buy7-(Diethylamino)-2H-chromen-2-one 2021, 86, 13111.
DOI: 10.1021/acs.joc.1c01380).
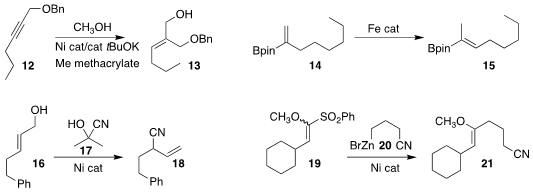
Wangqing Kong of Wuhan University observed high regio- and stereoselectivity in the
preparation of the allylic alcohol
13 by the addition of methanol to the propargyl ether 12
(Chem. Sci. 2021, 12, 9372.
DOI: 10.1039/D1SC02625A).
Yinlong Guo and Zheng Huang of the Shanghai Institute of Organic Chemistry also achieved high geometric
control in the isomerization
of the alkenyl borate 12 to the trisubstituted alkene 13
(ACS Catal. 2021, 11, 10138.
DOI: 10.1021/acscatal.1c02432).
Gui-Juan Cheng of the Chinese University of Hong Kong and Xianjie Fang of Shanghai Jiao Tong University
coupled the allylic alcohol 16 with acetone cyanohydrin 17 to give the α-branched nitrile 18
(ACS Catal. 2021, 11, 13880.
DOI: 10.1021/acscatal.1c03729).
Xia Zhang and Dawen Niu of Sichuan University found that coupling of the geometric mixture of enol ethers
19 with the organozinc 20 led to the enol ether 21 with high geometric control
(Chem. Commun. 2012, 57, 12273.
DOI: 10.1039/D1CC05347G).
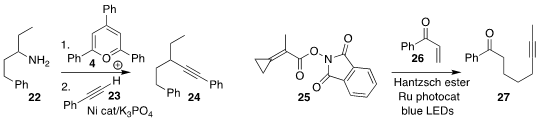
Xingjie Zhang and Guisheng Zhang of Henan Normal University
prepared the
alkyne 24 by coupling the pyridinium salt derived from the amine 22 with the alkyne 23
(Nature Commun. 2021, 12, 4904.
DOI: 10.1038/s41467-021-25222-1).
Yin Wei and Min Shi, also of the Shanghai Institute of Organic Chemistry, assembled the alkyne 27
by adding the acyloxyphthalimide 25 to the enone 26
(Chem. Sci. 2021, 12, 9088.
DOI: 10.1039/D1SC01889B).
Zhenyang Lin of the Hong Kong University of Science and Technology and Guosheng Liu
of the Shanghai Institute of Organic Chemistry prepared the trisubstituted
allene
29 in high ee by adding trimethylsilyl cyanide to the alkyne 28
(J. Am. Chem. Soc. 2021, 143, 14451.
DOI: 10.1021/jacs.1c07190).
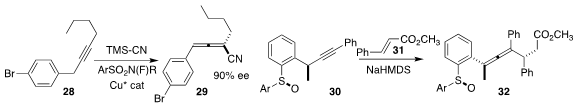
Rubén Sánchez-Obregón of the Universidad Nacional Autónoma de México assembled the
tetrasubstituted allene 32 by adding the alkyne 30 to ethyl cinnamate 31
(Tetrahedron Lett. 2021, 153425.
DOI: 10.1016/j.tetlet.2021.153425).
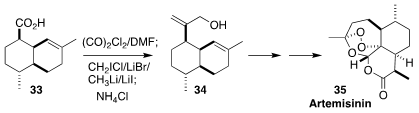
Artemisinin (35) is the key active component of current anti-malarials. In the
course of a synthesis of 35, K. N. Houk of UCLA and Quan Cai of Fudan University
used the Barluenga protocol to convert the acid 33 into the allylic alcohol 34
(Nature Catal. 2021, 4, 892.
DOI: 10.1038/s41929-021-00687-x).