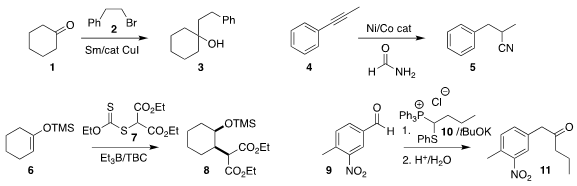
Yan Qi and Yongjun Liu of the Qingdao University of Science and Technology
demonstrated that the assembly of the tertiary alcohol 3 by the Barbier coupling
of cyclohexanone 1 with the bromide 2 could be effectively carried out with
Sm metal
(Chem. Commun. Histamine Data Sheet 2021, 57, 6169.
DOI: 10.1039/D1CC00965F).
Luo Yang of Xiangtan University observed that the hydrocyanation of the alkyne 4
proceeded with high regioselectivity, to give the
nitrile 5
(Adv. Synth. Price of 1530793-63-5 Catal. 2021, 363, 283.
DOI: 10.1002/adsc.202000935). PMID:24834360
Philippe Renaud of the University of Bern established conditions for the Giese addition
of 7 to the electron-rich enol ether 6, leading to the
diester 8
(Chem. Sci. 2021, 12, 2225.
DOI: 10.1039/D0SC06341J).
Jakob Magolan of McMaster University devised the
transpositive assembly of the ketone 11 by the addition of the easily-prepared
phosphonium salt 10 to the aldehyde 9, followed by hydrolysis
(Org. Lett. 2021, 23, 4548.
DOI: 10.1021/acs.orglett.1c01189).
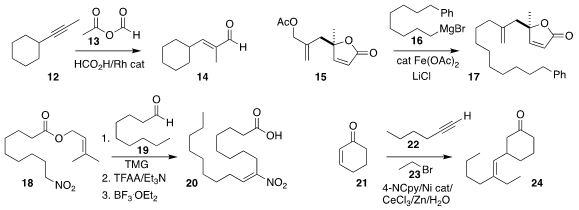
Kui-Ling Ding of the Shanghai Institute of Organic Chemistry and Qi-Lin Zhou
of Nankai University also observed high regioselectivity in the
hydroformylation
of 12 with 13 to give the unsaturated aldehyde 14
(Org. Lett. 2021, 23, 2074.
DOI: 10.1021/acs.orglett.1c00234).
Mathias Christmann of the Freie Universität Berlin found that the addition of
LiCl enabled the Fe-catalyzed coupling of the alkyl Grignard reagent 16 with the
allylic acetate 15, leading to 17
(Org. Lett. 2021, 23, 4731.
DOI: 10.1021/acs.orglett.1c01457).
Georg Manolikakes of the TU Kaiserlautern established conditions for the geometrically-controlled
construction of the nitro fatty acid 20, by the addition of the nitro ester 18
to the aldehyde 19, followed by dehydration and hydrolysis
(Eur. J. Org. Chem. 2021, 2239.
DOI: 10.1002/ejoc.202100247).
Wei Shu of the Southern University of Science and Technology
devised the Ni-catalyzed triply convergent assembly of the enone 21, the alkyne
22 and the bromide 23 to give the conjugate addition product 24
(Nature Commun. 2021, 12, 928.
DOI: 10.1038/s41467-021-21083-w).
Wei Deng and Jian-Nan Xiang of Hunan University and Yong-Yue Luo of the
Chinese Academy of Tropical Agricultural Sciences established the
decarbonylative coupling of the aldehyde 26 with the tertiary propargylic
alcohol 25 to give the ketone 27
(Org. Biomol. Chem. 2021, 19, 3154.
DOI: 10.1039/D1OB00212K).
Pengchen Qian and Jiang Cheng of Wenzhou University reported a closely-related investigation
(Org. Biomol. Chem. 2021, 19, 2416.
DOI: 10.1039/D1OB00192B).
Brian Gold of the University of New Mexico and Ronald T. Raines of MIT described the
preparation of the reactive dibenzocyclooctyne 29 by the carbodiimide-mediated
dehydration of the tetrazole 28
(J. Am. Chem. Soc. 2021, 143, 9489.
DOI: 10.1021/jacs.1c03133).
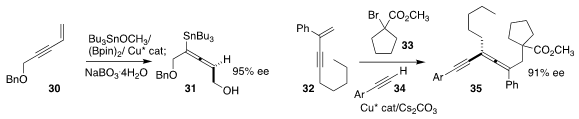
Min Wang and Jian Liao of the Chengdu Institute of Biology and Lianrui Hu of
Xihua University used an enantiomerically-pure sulfoxide to mediate the
enantioselective conversion of the enyne 30 to the stannyl
allene 31
(Chem. Sci. 2021, 12, 3032.
DOI: 10.1039/D0SC05425A).
Xin-Yuan Liu, also of the Southern University of Science and
Technology, found that a Cinchona-derived Cu complex directed the
enantioselective assembly of the allene 35 by the triply-convergent coupling of
the enyne 32, the α-bromoester 33, and the alkyne 34
(Angew. Chem. Int. Ed. 2021, 60, 2160.
DOI: 10.1002/anie.202013022).
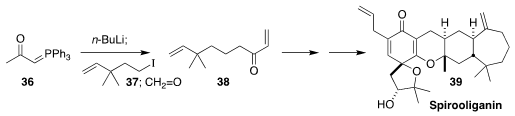
Spirooliganin (39), isolated from the stem bark of Illicium oligandrum, showed
effective antiviral activity. Pursuing the synthesis of 39, Kendall N. Houk of
UCLA and Jing Qu and Shi-Shan Yu of the Institute of Material Medica prepared
the starting enone 38 by coupling the useful linchpin phosphonium salt first
with the iodide 37, then with formaldehyde
(Chem. Sci. 2021, 12, 7003.
DOI: 10.1039/D1SC01277K).