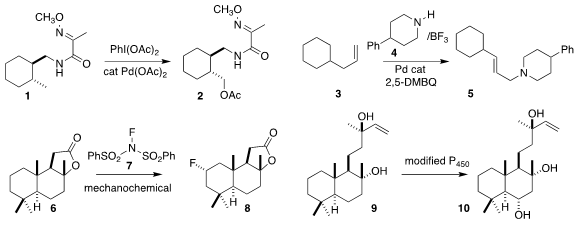
Qingtao Meng and Zhiqiang Zhang of the University of Science and Technology
Liaoning and Yu-Peng He of the Dalian University of Technology used the amide of
1 to direct acetoxylation, leading to the ester 2
(J. Org. Chem. 2022, 87, 6378.
DOI: 10.1021/acs.joc.2c00085).
M. Christina White of the University of Illinois assembled the allylic
amine 5 by coupling the alkene 3 with the amine 4
(Science 2022, 376, 276.
DOI: 10.1126/science.abn8382).
Soon Hyeok Hong of KAIST used mechanical grinding with the fluorosulfonamide 7 to convert the lactone 6 to the fluorinated product 8
(Adv. Synth. Cat. PMID:24268253 5-Azidopentan-1-amine Order 2022, 364, 1975.
DOI: 10.1002/adsc.202200206).
Hans Renata of Scripps Florida used a mutated P450 to oxidize the diol 9
to the triol 10
(J. Am. Chem. Soc. 2022, 144, 7616.
DOI: 10.1021/jacs.2c02958).
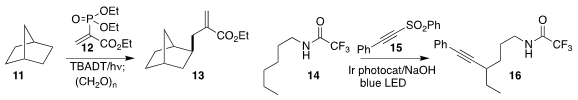
Timothy Noël of the University of Amsterdam used a decatungstate
photocatalyst to couple 11 with the alkenyl phosphate 12, leading to the ester
13
(Chem. Formula of 1251005-61-4 Sci. 2022, 13, 7325.
DOI: 10.1039/D2SC01581A).
Fei Pan of Sichuan Normal University assembled
the alkyne 16 by coupling the amide 14 with the alkynyl sulfone 15
(Chem. Commun. 2022, 58, 2295.
DOI: 10.1039/D1CC06885G).
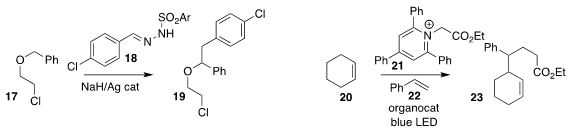
Xihe Bi of Northeast Normal University observed high
regioselectivity in the formation of 19 by the the insertion of the carbene
derived from 18 into 17
(Nature Commun. 2022, 13, 1674.
DOI: 10.1038/s41467-022-29323-3).
Paolo Melchiorre of
ICIQ used a Binol-derived thiophosphoric acid to mediate the preparation of the
ester 23 by the three-component coupling of the alkene 20, the pyridinium salt
21, and styrene 22
(J. Am. Chem. Soc. 2022, 144, 1113.
DOI: 10.1021/jacs.1c11712).
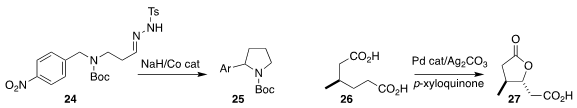
Yoshihiro Matano of Niigata University cyclized the tosylhydrazone 24 to the
pyrrolidine 25, via the intermediate diazo alkane
(Org. Lett. 2022, 24, 3839.
DOI: 10.1021/acs.orglett.2c01411).
Jin-Quan Yu of Scripps La Jolla achieved high diastereoselectivity in the
cyclization of the diacid 25 to the lactone 26
(Science 2022, 376, 1481.
DOI: 10.1126/science.abq3048).
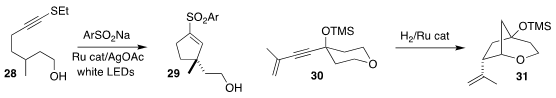
Gangguo Zhu of Zhejiang Normal University cyclized the alkyne 28 to the
cyclopentene 29
(Angew. Chem. Int. Ed. 2022, 61, e202110864.
DOI: 10.1002/anie.202110864).
Alois Fürstner of
the Max-Planck-Institut Mülheim generated an intermediate Ru carbene from the
enyne 30, that cyclized to the cyclopentane 31
(J. Am. Chem. Soc. 2022, 144, 4158.
DOI: 10.1021/jacs.1c13446).
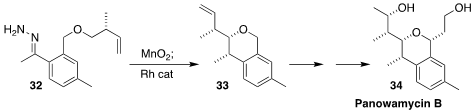
In the course of a screening program for the treatment of tropical diseases,
panowamycin B (34) was isolated from a culture of Streptomyces sp. K07-0010. Jared
T. Shaw of the University of California, Davis constructed the isochroman
skeleton of 34 by the Rh catalyzed diastereoselective cyclization to 33 of the
diazo alkane derived from the hydrazone 32
(Angew. Chem. Int. Ed. 2022, 61, e202203072.
DOI: 10.1002/anie.202203072).