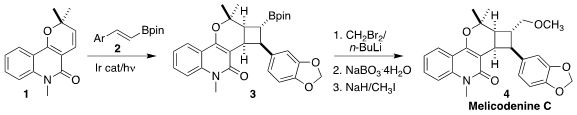
Melicodenine C (4) was isolated from the leaves of Melicope
denhamii, a rutaceous shrub found in Borneo that has been used in indigenous
medicine. PMID:23892407 John R. Swierk of Binghamton University and M. Kevin Brown of Indiana
University constructed the
cyclobutane ring of 4
by photochemical cycloaddition of the pyridone 1 with the
alkenyl boronate 2 to give 3
(Angew. Chem. Int. 2-(1H-Pyrazol-3-yl)propan-2-ol manufacturer Ed. 2022, 61, e202200725.
DOI: 10.1002/anie.202200725).
Myrioneurinol (7), isolated from Myrioneuron nutans, a small
tree of north Vietnam, showed significant antimalarial activity. Buy138517-61-0 Zhiqiang Ma of
the South China University of Technology established the
cyclohexenone of 6
by silver-mediated intramolecular [2+2] cycloaddition of the alkyne 5, followed by acid-mediated rearrangement
(Angew. Chem. Int. Ed. 2022, 61, e202200085.
DOI: 10.1002/anie.202200085).
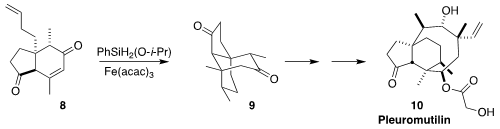
Pleuromutilin (10) is the parent of a family of potent and
commercially-important antibiotics. Sergey V. Pronin of the University of California,
Irvine assembled the tricyclic ketone 9 by the radical cyclization of 8
(J. Am. Chem. Soc. 2022, 144, 10174.
DOI: 10.1021/jacs.2c04708).
Richmond Sarpong of the University of California, Berkeley used a
similar strategy in a recent synthesis of longiborneol (not illustrated)
(Nature Chem. 2022, 14, 456.
DOI: 10.1038/s41557-021-00870-4).
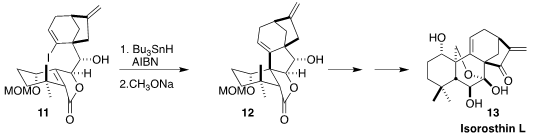
Isorosthin L (13) was isolated from Isodon rosthornii, a perennial herb used to treat rheumatism and sore
throats. En route to 13, Guangxin Liang of ShanghaiTech University cyclized the
iodoalkene 11 to the pentacyclic 12
(Angew. Chem. Int. Ed. 2022, 61, e202114489.
DOI: 10.1002/anie.202114489).
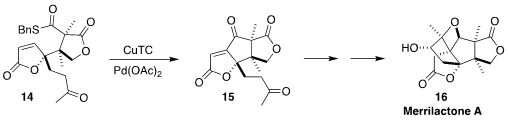
Although some of the Illicium sesquiterpenes promote neurite outgrowth
in cortical neuron cell culture, merrilactone A (16) was recently shown to
be inactive. Ryan A. Shenvi of Scripps/La Jolla closed the central
cyclopentane of
16 by the oxidative cyclization of the thioester 14 to 15
(Angew. Chem. Int. Ed. 2022, 61, e202114514.
DOI: 10.1002/anie.202114514).
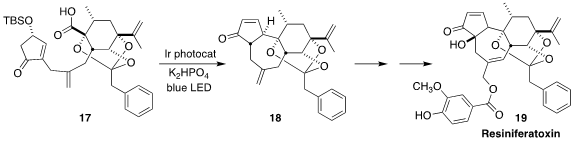
Resiniferatoxin (19), isolated from the latex of Euphorbia
resinifera, has found application as a neuroblocking agent. Masayuki Inoue
of the University of Tokyo closed the central
cycloheptane of 19 by the
decarboxylative radical cyclization of 17 to 18
(Org. Lett. 2022, 24, 929.
DOI: 10.1021/acs.orglett.1c04286).