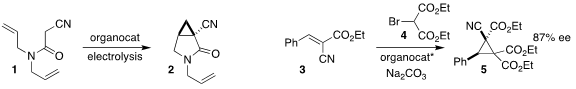
Hai-Chao Xu of Xiamen University used a phenothiazine-based catalyst to
mediate the oxidative cyclization of the amide 1 to the
cyclopropane 2
(J. Am. Chem. Soc. 2022, 144, 2343.
DOI: 10.1021/jacs.1c12762).
Zsolt Rapi of the Budapest University of Technology
and Economics showed that a carbohydrate-derived crown ether effectively
directed the addition of 4 to 3, leading to the cyclopropane 5
(Eur. 58349-17-0 web J. Org. Chem. 2022, e202200112.
DOI: 10.1002/ejoc.202200112). 1203499-17-5 uses
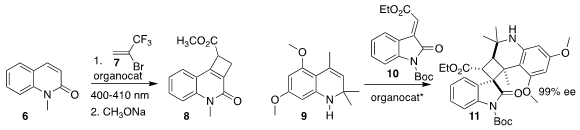
Hao Guo of Fudan University showed that a thioxanthone photocatalyst promoted
the addition of the trifluoromethyl-substituted alkene 7 to the quinolone
6, to give an adduct that was carried on to the
cyclobutenecarboxylate 8
(Tetrahedron Lett. 2022, 92, 153673.
DOI: 10.1016/j.tetlet.2022.153673).
Qin Ouyang of the Third Military Medical University and
Xu Tian of Guangzhou Medical University demonstrated that a BINOL-derived
phosphoric acid directed the combination of the
dihydroquinoline 9 with the
oxindole 10, leading to the
cyclobutane 11
(Org. Chem. PMID:24883330 Front. 2022, 9, 1621.
DOI: 10.1039/D1QO01708J).
Liang Xu of Shihezi University and Pengfei Li of Xi’an Jiaotong University used a
pinene-derived diborane in combination with a substituted pyridine to promote
the addition of the cyclopropyl ketone 13 to the alkene 12, leading to the
cyclopentane 14
(J. Am. Chem. Soc. 2022, 144, 8870.
DOI: 10.1021/jacs.2c03673).
Wei Huang and Qian Zhao of
the Chengdu University of Traditional Chinese Medicine showed that the
Jørgenson-Hayashi catalyst effectively directed the assembly of the cyclopentane
17 by the addition of the oxindole 15 to cinnamaldehyde 16
(Eur. J. Org. Chem. 2022, e202200489.
DOI: 10.1002/ejoc.202200489).
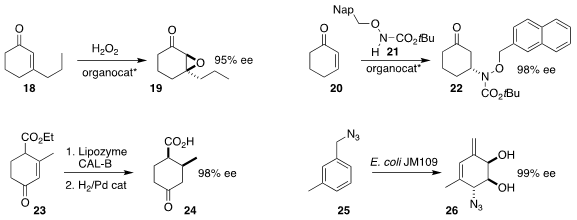
Katharina Bica-Schröder of TU Wien also used a chiral phosphoric acid to
epoxidize the cyclohexenone 18, leading to 19
(Angew. Chem. Int. Ed. 2022, 61, e202202189.
DOI: 10.1002/anie.202202189).
Hyeung-geun Park of Seoul National University employed a
Cinchona-derived urea to promote the addition of the alkoxy amine 21 to
cyclohexenone 20, leading to the amine 22
(Org. Lett. 2022, 24, 1647.
DOI: 10.1021/acs.orglett.2c00192).
William P. Gallagher and Francisco González-Bobes of Bristol Myers Squibb developed the
equilibrating enantioselective
saponification
of the inexpensive Hagemann’s ester (23), leading to the acid 24
(Org. Process Res. Dev. 2022, 26, 583.
DOI: 10.1021/acs.oprd.1c00339).
Nicolás Veiga and Ignacio Carrera of the Universidad de la República showed that
enzymatic oxidation of the benzylic azide 25 to the dihydrodiol led, after
Winstein rearrangement, to the diene 26
(Eur. J. Org. Chem. 2022, e202101156.
DOI: 10.1002/ejoc.202101156).
Samuel H. Gellman of the University of Wisconsin created a foldamer that
catalyzed the dimerization of the dialdehyde 27 to the macrocycle 28
(J. Am. Chem. Soc. 2022, 144, 2225.
DOI: 10.1021/jacs.1c11542).
Elizabeth H. Krenske of the University of
Queensland and Pauline Chiu of the University of Hong Kong cyclized the epoxy
triene 29 to the bicyclic ketone 30 with high relative and absolute
stereocontrol
(Angew. Chem. Int. Ed. 2022, 61, e202116099.
DOI: 10.1002/anie.202116099).
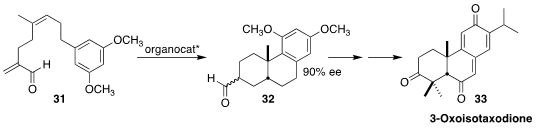
3-Oxoisotaxodione (33) was isolated from the Chinese coffin tree Taiwania
cryptomeriodes. James L. Gleason of McGill University established the relative
and absolute configuration of 33 by the urea-catalyzed cyclization of the diene
31 to the tricyclic aldehyde 32
(Org. Lett. 2022, 24, 2305.
DOI: 10.1021/acs.orglett.2c00444).