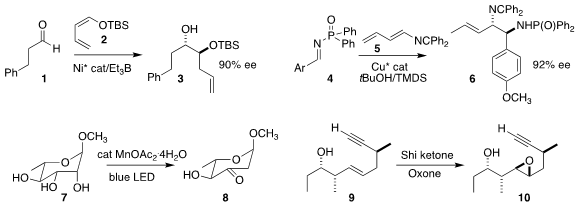
Alois Fürstner of the Max-Planck-Institut für Kohlenforschung set the
relative and absolute configuration of 3 by the Ni-mediated addition of the enol
ether 2 to the aldehyde 1
(J. Am. Chem. Soc. 2021, 143, 13489.
DOI: 10.1021/jacs.1c07042).
Depending on the choice of Cu catalyst, Xinxin Shao of Hangzhou Normal University and Steven J.
Malcolmson of Duke University could combine the imine 4 with the diene 5 to give
either diastereomer of the protected
diamine 6
(J. Am. Chem. PMID:25105126 Soc. 2021, 143, 13999.
DOI: 10.1021/jacs.1c07707).
Alison E. Wendlandt of MIT used visible light to promote the conversion
of the sugar 7 to the ketone 8
(J. Am. 240401-09-6 manufacturer Chem. Soc. Price of 1-Methylcyclobutanecarboxylic acid 2021, 143, 13798.
DOI: 10.1021/jacs.1c05993).
Mark S. Taylor of the University of Toronto reported a parallel investigation
(ACS Catal. 2021, 11, 11171.
DOI: 10.1021/acscatal.1c03050).
Michael J. Krische of the University of Texas used the
Shi
ketone to achieve high diastereoselectivity in the conversion of the alcohol
9 to the epoxide 10
(Angew. Chem. Int. Ed. 2021, 60, 13923.
DOI: 10.1002/anie.202103845).
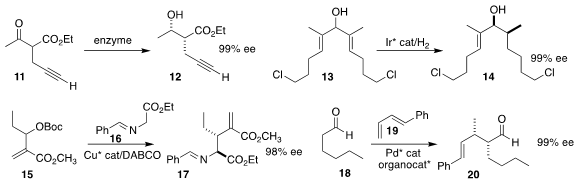
Samik Nanda of the Indian Institute of Technology Kharagpur effected
enzymatic reduction of the racemic β-ketoester
11 to the alcohol
12 on a twenty-gram scale
(Tetrahedron 2021, 94, 132356.
DOI: 10.1016/j.tet.2021.132356).
Pher G. Andersson of Stockholm University effected the enantioselective
reduction of the diene 13 to the alcohol 14
(Angew. Chem. Int. Ed. 2021, 60, 19428.
DOI: 10.1002/anie.202107267).
Xianghong Hao and Hongchao Guo of the
China Agricultural University assembled the diester 17 by adding the
protected glycine 16 to the
Morita-Baylis-Hillman adduct 15
(Chem. Commun. 2021, 57, 8059)
DOI: 10.1039/D1CC02861H).
Weiwei Zi of Nankai University used a Pd catalyst combined with the Hayashi-Jørgensen amine
to mediate the addition
of the aldehyde 18 to the diene 19, enabling controlled access
to each of the enantiomerically-pure diastereomers of the aldehyde 20
(J. Am. Chem. Soc. 2021, 143, 10948)
DOI: 10.1021/jacs.1c02220).
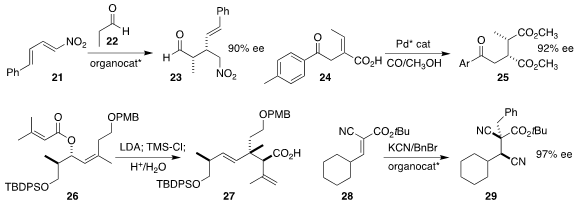
Katsuhiko Moriyama of Chiba University devised a diamine catalyst that
directed the addition of propionaldehyde 22 to the nitrodiene 21, leading the
aldehyde 23
(Chem. Commun. 2021, 57, 11457.
DOI: 10.1039/D1CC04453B).
Kaiwu Dong of East China Normal University observed high enantioselectivity and diastereoselectivity in the
carbonylation of the unsaturated acid 24 to give the diester 25
(Angew. Chem. Int. Ed. 2021, 60, 17693.
DOI: 10.1002/anie.202105977).
David R. Williams of Indiana University achieved high
diastereoselectivity in the
Claisen rearrangement of the ester
26 to the acid 27
(Tetrahedron 2021, 95, 132354.
DOI: 10.1016/j.tet.2021.132354).
Takashi Ooi of Nagoya University developed an
alternative strategy for the assembly of acyclic quaternary centers, using a
triazolium salt to direct the addition of cyanide ion to the unsaturated ester
28, then alkylating the product to give 29
(J. Am. Chem. Soc. 2021, 143, 11218.
DOI: 10.1021/jacs.1c05380).
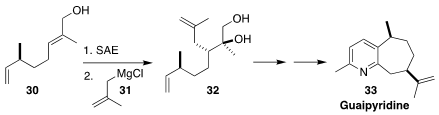
Guaipyridine (33) was isolated from the root of Cyperus scariosus, a perennial
herbaceous plant of Australia and New Guinea. Haji Akber Aisa of the Xinjiang
Technical Institute of Physics and Chemistry set the relative and absolute
configuration of 33 by
Sharpless asymmetric epoxidation of the allylic alcohol
30, followed by opening with methallyl magnesium chloride 31 to give 32
(Org. Biomol. Chem. 2021, 19, 7081.
DOI: 10.1039/D1OB01299A).