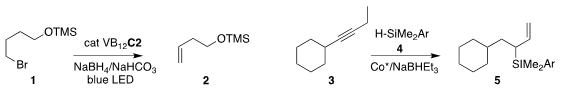
Julian G. 61881-19-4 web West of Rice University used a vitamin B12-based catalyst to
prepare the terminal alkene 2 by the elimination of the primary bromide
1
(Chem. PMID:31085260 Sci. 2021, 12, 1736.
DOI: 10.1039/D0SC05925K).
Jung-Woo Park of KAIST assembled the
allyl silane 5 by
coupling the internal alkyne 3 with the trialkylsilane 4
(ACS Catal. 2021, 11, 1548.
DOI: 10.1021/acscatal.0c05424).
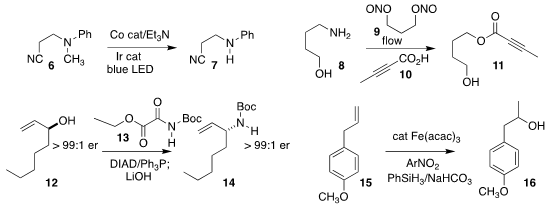
Daniele Leonori of the University of Manchester effected the selective oxidative
demethylation of the tertiary
amine 6 to give the secondary amine 7
(Angew. Chem. Int. Ed. 5-Bromo-2-cyclopropoxypyridine Order 2021, 60, 7669.
DOI: 10.1002/anie.202100051).
Hélène Lebel of the Université de Montréal used
a flow system with the dinitrite 9 to couple the acid 10 with the diazonium salt
derived from the primary amine 8, leading to the ester 11
(Chem. Commun. 2020, 56, 10938.
DOI: 10.1039/D0CC03242E).
Jonathan Clayden of the University of Bristol showed that the
Mitsunobu
coupling of N-Boc ethyl oxamate 13 with the allylic alcohol 12 followed
by hydrolysis led to the amine 14 with clean inversion of absolute configuration
(J. Org. Chem. 2021, 86, 8538.
DOI: 10.1021/acs.joc.1c00918).
Armido Studer of Westfalische Wilhelms-Universität devised the iron-catalyzed Markovnikov hydration of the
terminal alkene 15 to the secondary alcohol 16
(Angew. Chem. Int. Ed. 2021, 60, 8313.
DOI: 10.1002/anie.202015740).
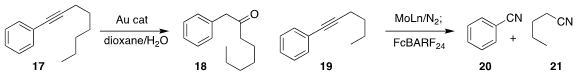
Andrey F. Asachenko of the A. V. Topchiev Institute of Petrochemical Synthesis optimized the selective
hydration of the internal aryl alkyne 17 to
the benzyl ketone 18
(Chem. Commun. 2021, 57, 5686.
DOI: 10.1039/D1CC01837J).
Qian Liao of the Dalian University of Technology and Nicolas Mézailles of the Université Paul Sabatier
exposed a Mo complex to N2, then used that to split the internal alkyne 19 into
the two nitriles 20 and 21
(Angew. Chem. Int. Ed. 2021, 60, 12242.
DOI: 10.1002/anie.202015183).
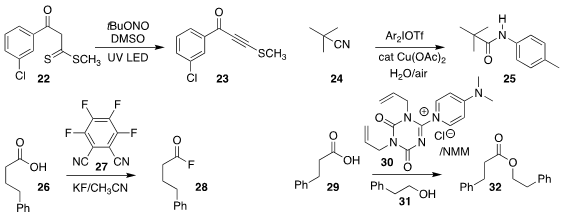
Maya Shankar Singh of Banaras Hindu University prepared the
alkynyl sulfide
23 by the net elimination of H2S from the β-oxodithioester 22
(J. Org. Chem. 2021, 86, 5908.
DOI: 10.1021/acs.joc.1c00417).
Hui-cheng Cheng and Jiao-li Ma of the Guangdong University of Petroleum Technology showed
that arylation of the nitrile 24 led to the amide 25
(Tetrahedron Lett. 2021, 71, 153048.
DOI: 10.1016/j.tetlet.2021.153048).
Haoran Sun of the University of South
Dakota used 27 to convert the carboxylic acid 26 to the
acid fluoride 28
(J. Org. Chem. 2021, 86, 6066.
DOI: 10.1021/acs.joc.0c02491).
Akira Matsumoto and Keiji Maruoka of Kyoto University reported that
Selectfluor can also be used to effect this transformation
(J. Org. Chem. 2021, 86, 5401.
DOI: 10.1021/acs.joc.1c00188).
Munetaka Kunishima of Kanazawa
University developed the reagent 30 for preparing the ester 32 by coupling the
acid 29 with the alcohol 31
(Org. Biomol. Chem. 2021, 19, 4712.
DOI: 10.1039/D1OB00450F).
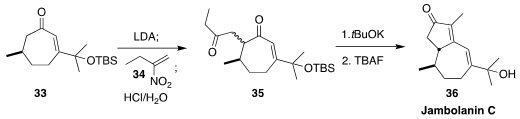
Thomas Gerard Minehan of California State University, Northridge showed that
addition of the enolate from 33 to the nitroalkene 34 gave an intermediate that
on acid hydrolysis was converted to the diketone 35. Intramolecular
aldol condensation followed by
desilylation then completed the synthesis of jambolanin C (36)
(J. Org. Chem. 2021, 86, 3074.
DOI: 10.1021/acs.joc.0c02747).