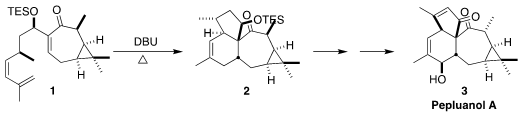
Pepluanol A (3) was isolated from the European milkweed Euphorbia
peplus. 957476-07-2 uses BuyLumisterol 3 (>90%) Tanja Gaich of the University of Konstanz devised a route to 3
based on the intramolecular
Diels-Alder
cycloaddition of the cis diene 1, leading to the tetracylic 2
(J. Am. PMID:26446225 Chem. Soc. 2021, 143, 11934.
DOI: 10.1021/jacs.1c05257).
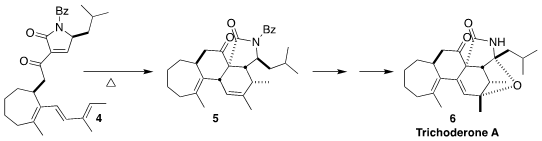
Bastien Nay of the Ecole Polytechnique devised the cyclization of the
tetraene 4 to the lactam 5
(Org. Lett. 2021, 23, 5755.
DOI: 10.1021/acs.orglett.1c01922).
The tricycle 5 had already been oxidized to trichoderone A (6)
by Pema-Tenzin Puno and Jun Deng of the Kunming Institute of Botany
(Angew. Chem. Int. Ed. 2021, 60, 15963.
DOI: 10.1002/anie.202102831).
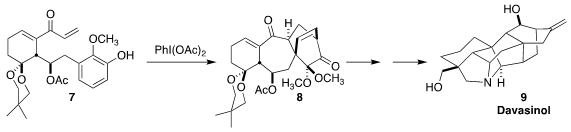
The hetesine-type alkaloid davasinol (9) was isolated from Delphinium davisii
Munz of Turkey. En route to 9, Hanfeng Ding of Zhejiang University oxidized the aromatic
ring of 7 to a cyclohexadienone that cyclized to the diketone 8
(J. Am. Chem. Soc. 2021, 143, 10576.
DOI: 10.1021/jacs.1c05703).
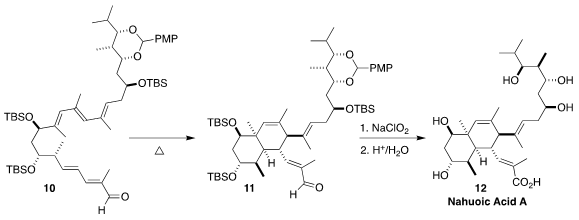
Nahuoic acid A (12) was isolated via a chemical genetics approach from a
Streptomyces sp. derived from a marine sediment. The bicyclic 12 is the
first reported cofactor-competitive inibitor of the epigenetic enzyme SETD8.
Rosana Alvarez and Angel R. de Lera of the Universidade de Vigo synthesized
12 by way of the internal Diels-Alder cyclization of the pentaene 10
to the aldehyde 11
(Chem. Sci. 2021, 12, 15157.
DOI: 10.1039/D1SC04524E).
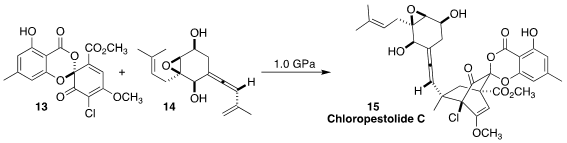
Chloropestolide C (15) was isolated from a scale-up fermentation of
Pestalotiopsis fici. Following a biomimetic proposal, Takahiro Suzuki of
Hokkaido University showed that under high pressure, the conjugated alkene of 14
added to the diene 13, leading to 15
(J. Org. Chem. 2021, 86, 15597.
DOI: 10.1021/acs.joc.1c02108).
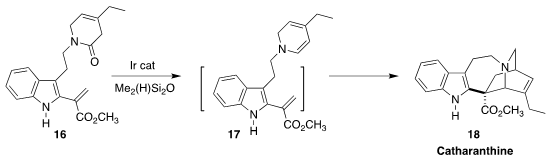
Darren J. Dixon of the University of Oxford developed a strategy for the
reduction of a dihydropyridone 16 to the dihydropyridine 17 – the
long-sought biosynthetic intermediate dehydrosecodine. The isolated product from the
reaction was the Vinca alkaloid catharanthine
(J. Am. Chem. Soc. 2021, 143, 10828.
DOI: 10.1021/jacs.1c04980).