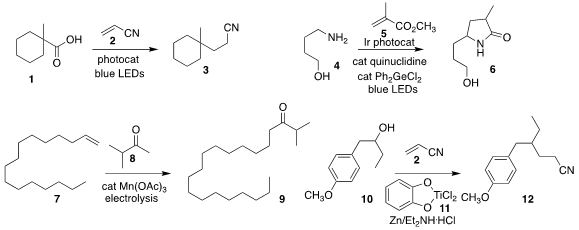
Yasuharu Yoshimi of the University of Fukui devised a photocatalyst that
efficiently promoted the coupling of the acid 1 with acrylonitrile 2,
to give, with the net addition of two carbons,
the nitrile 3
(J. Org. Chem. Benzyl (2-aminoethyl)carbamate Purity 2022, 87, 7405.
DOI: 10.1021/acs.joc.2c00643).
Kounosuke Oisaki and Motomu Kanai of the University of
Tokyo added a germanium catalyst to promote the coupling of the primary amine 4
with methyl methacrylate 5, leading to the
γ-lactam 6
(Org. Lett. Propargyl-PEG5-acid site 2022, 24, 3325.
DOI: 10.1021/acs.orglett.2c00871).
Julien C. Vantourout of the Université Lyon 1 showed that the
coupling of the alkene 7 with
the ketone 8 to give 9 could be driven by electrolysis, making the
Mn(OAc)3 catalytic
(J. Org. PMID:23991096 Chem. 2022, 87, 5690.
DOI: 10.1021/acs.joc.2c00054).
Takuya Suga and Yutaka Ukaji of Kanazawa University activated the alcohol 10 with the Ti complex
11, to give an intermediate that could be added in a
conjugate sense to acrylonitrile 2, leading to the nitrile 12
(Angew. Chem. Int. Ed. 2022, 61, e202112533.
DOI: 10.1002/anie.202112533).
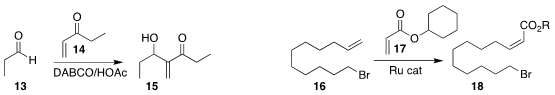
Giovanni W. Amarante of the Federal University of Juiz de Fora and Fernando
Coelho of the University of Campinas developed an improved protocol for the
Morita-Baylis-Hillman coupling, allowing the assembly of the alcohol
15 by the addition of the enone 14 to the aldehyde 13
(Eur. J. Org. Chem. 2022, e202101448.
DOI: 10.1002/ejoc.202101448).
The late Robert H. Grubbs of Caltech developed a bulky Ru catalyst that effected
the Z-selective
cross metathesis of the acrylate 17 with the alkene 16, to give 18
(Angew. Chem. Int. Ed. 2022, 61, e202113089.
DOI: 10.1002/anie.202113089).
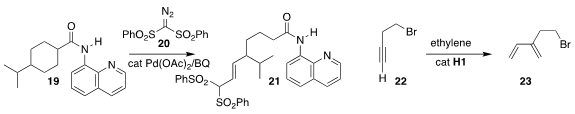
Ming Yan of Sun Yat-sen University devised the coupling with 20 and subsequent
fragmentation that opened the carboxamide 19 to the alkene 21
(Org. Lett. 2022, 24, 536.
DOI: 10.1021/acs.orglett.1c03952).
Aaron D. Sadow of Iowa State University optimized the
enyne metathesis of the alkyne
22 with ethylene
to give the diene 23
(ACS Catal. 2022, 12, 226.
DOI: 10.1021/acscatal.1c04074).
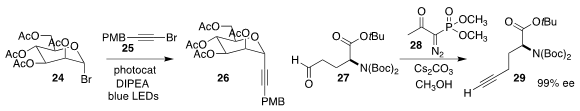
Shouyun Yu of Nanjing University coupled the glycosyl bromide 24 with the
bromoalkyne 25 to give the C-alkynyl glycoside 26
(Org. Lett. 2022, 24, 364.
DOI: 10.1021/acs.orglett.1c04041).
Isaac J. Krauss of Brandeis University devised conditions for the non-epimerizing
conversion of the aldehyde 27 with the
Ohira reagent
28 to the alkyne 29
(J. Org. Chem. 2022, 87, 3841.
DOI: 10.1021/acs.joc.1c03027).
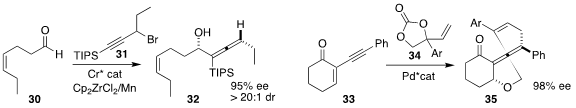
Zhaobin Wang of Westlake University assembled the enantiomerically-enriched
allene
32 by the addition of the racemic propargylic bromide 31 to the aldehyde
30
(Angew. Chem. Int. Ed. 2022, 61, e202117114.
DOI: 10.1002/anie.202117114).
Liang-Qiu Lu of Central China Normal University combined the enone 33 with the
cyclic carbonate 34 to give the allene 35
(Angew. Chem. Int. Ed. 2022, 61, e202117215.
DOI: 10.1002/anie.202117215).
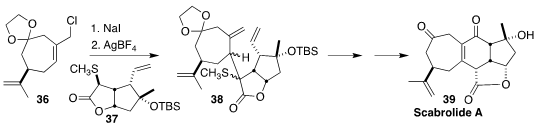
Scabrolide A (39), isolated from the soft coral Sinularia scabra, inhibits both
IL-6 and IL-12, and shows only moderate cyotoxicity. Alois Fürstner of the
Max-Planck-Institut für Kohlenforschung devised a convergent route to 39, based
on the coupling of the allylic chloride 36 with the sulfide 37 to give
38
(J. Am. Chem. Soc. 2022, 144, 1528.
DOI: 10.1021/jacs.1c12401).