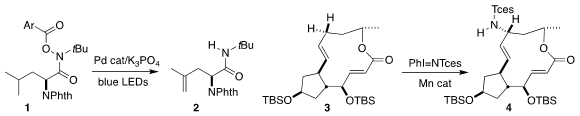
Shouyun Yu of Nanjing University devised a protocol for the distal
dehydrogenation of the acyloxyamide 1 to the alkene 2
(Org. Lett. 2021, 23, 6931.
DOI: 10.1021/acs.orglett.1c02509).
Armido Studer of Westfalische Wilhelms-Universität reported related results
(Chem. Eur. 2227206-09-7 structure J. 2021, 27, 16621.
DOI: 10.1002/chem.202103509).
M. Christina White of the University of Illinois achieved remarkable selectivity in the
allylic amination of the
protected brefeldin A (3), leading to 4
(J. Am. Chem. Xphos Pd G4 Order Soc. PMID:23891445 2021, 143, 14969.
DOI: 10.1021/jacs.1c06335).
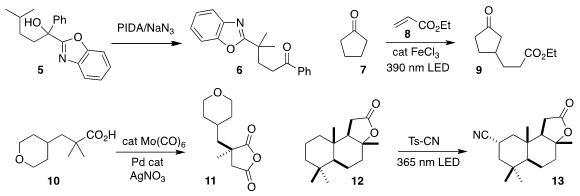
Yahong Li and Chen Zhu of Soochow University effected the oxidative
rearrangment of the tertiary alcohol 5 to the aryl ketone 6
(Org. Chem. Front. 2021, 8, 6395.
DOI: 10.1039/D1QO01209F).
Tomislav Rovis of Columbia University assembled the ester 9 by adding
cyclopentanone
7 to ethyl acrylate 8
(Synlett 2021, 32, 1767.
DOI: 10.1055/s-0040-1720388).
Jin-Quan Yu of Scripps/La Jolla selectively carbonylated the methyl group of 10,
to give the easily-differentiated anhydride 11
(Angew. Chem. Int. Ed. 2021, 60, 16382.
DOI: 10.1002/anie.202104645).
Soon Hyeok Hong of KAIST converted sclareolide (12) to the equatorial
nitrile 13
(Org. Lett. 2021, 23, 5501.
DOI: 10.1021/acs.orglett.1c01846).
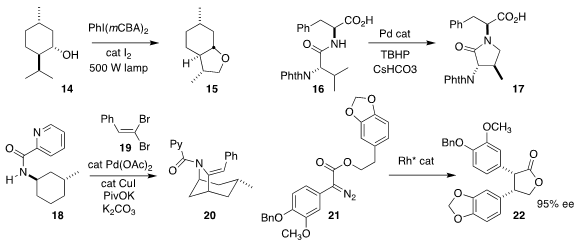
Stefan Bräse of the Karlsruhe Institute of Technology oxidized menthol (14) to
the tetrahydrofuran 15
(Eur. J. Org. Chem. 2021, 3478.
DOI: 10.1002/ejoc.202100652).
Professor Yu cyclized the amide 16 to the
lactam 17
(J. Am. Chem. Soc. 2021, 143, 21657.
DOI: 10.1021/jacs.1c10183).
Bert U. W. Maes of the University of Antwerp assembled the bridged
pyrrolidine 20 by
combining the amide 18 with the dibromide 19
(Angew. Chem. Int. Ed. 2021, 60, 21988.
DOI: 10.1002/anie.202106716).
Takumi Furuta of Kyoto Pharmaceutical University achieved high ee in the
cyclization of the α-aryl α-diazo ester 21 to the
lactone 22
(ACS Catal. 2021, 11, 568.
DOI: 10.1021/acscatal.0c03689).
Guozhu Zhang of the Shanghai Institute of Organic Chemistry constructed the
cyclobutanol
25 by adding the iodomethyl silane 24 to the alkyne 23
(Nature Commun. 2021, 12, 6404.
DOI: 10.1038/s41467-021-26670-5).
X. Peter Zhang of Boston College used a Co catalyst to
cyclize the α-aryl α-diazo ketone 26 to the
cyclobutanone 27
(J. Am. Chem. Soc. 2021, 143, 11670.
DOI: 10.1021/jacs.1c04968).
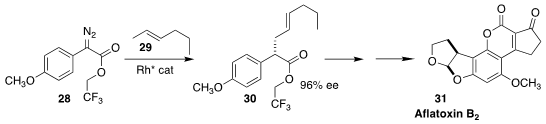
The aflatoxins, exemplified by aflatoxin B2 (31), are a family of dangerous
liver carcinogens produced by Aspergillus fungi. Huw M. L. Davies of Emory
University and Erik J. Sorensen of Princeton University established the key
stereogenic center of 31 by the enantioselective insertion of the α-aryl α-diazo
ester 28 into the methyl group of the alkene 29 to give 30
(Org. Lett. 2021, 23, 9393.
DOI: 10.1021/acs.orglett.1c03502).