Aristoquinoline (2), isolated from the leaves of the Maqui tree
Aristotelia chilensis, was found to be a nicotinic acetylcholine receptor
antagonist. BuyMethyl 6-aminopicolinate Buy130473-38-0 Keith P. Reber of Towson University assembled 2 by a
Bischler-Napieralski type cyclization of the activated amide 1, followed by reduction
(Synthesis 2022, 54, 1404.
DOI: 10.1055/s-0041-1737276).
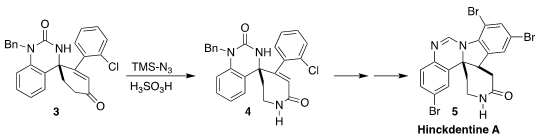
Hinckdentine A (5) was isolated from the bryozoan Hincksinoflustra
denticulata collected on the eastern coast of Tasmania. PMID:23563799 En route to 5,
Zhishan Su of Sichuan University and Ran Hong of the Shanghai Institute of
Organic Chemistry effected the ring expansion of the
cyclohexenone
3 to the lactam 4
(JACS Au 2022, 2, 793.
DOI: 10.1021/jacsau.2c00048).
Madangamine E (8) is one of several alkaloids isolated from the marine
sponge Xestospongia ingens van Soest. Trevor A. Hamlin of the Vrije
Universiteit Amsterdam and Darren J. Dixon of the University of Oxford showed that a highly enantioselective thiourea-catalyzed intramolecular
Michael cyclization
of the nitroalkene 6 to the ketone 7 enabled the asymmetric total synthesis of
8
(J. Am. Chem. Soc. 2022, 144, 1407.
DOI: 10.1021/jacs.1c12040).
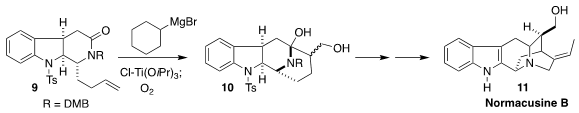
Normacusine B (11), also known as vellosiminol, is a hypotensive
alkaloid isolated from the root of Strychnos atlantica. Fei Xue and Yong
Qin, also of Sichuan University, established the second
piperidine ring of 11
by the Ti-mediated oxidative cyclization of the amide 9 to the diol 10
(Org. Lett. 2022, 24, 3515.
DOI: 10.1021/acs.orglett.2c01177).
Daphenylline (14) is one of a variety of the several related alkaloids
isolated from the genus Daphniphyllum, evergreen shrubs and trees of east
and southeast Asia long used in medicinal chemistry. A key step in the total
synthesis of 14 described by Hai-Hua Lu of Westlake University is the
diastereoselective double
reductive amination
of the keto aldehyde 12, that was followed by spontaneous cyclization to
the lactam 13
(J. Am. Chem. Soc. 2022, 144, 5750.
DOI: 10.1021/jacs.2c01674).
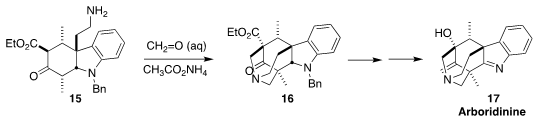
Arboridine (17) was isolated from a Malaysian Kopsia species.
Zhiqiang Ma of South China University of Technology assembled the
pentacyclic intermediate 16 by the double intramolecular
Mannich cyclization of 15
(J. Org. Chem. 2022, 87, 8223.
DOI: 10.1021/acs.joc.2c00602).