Christopher Uyeda of Purdue University reduced 1 to the intermediate
vinylidene (also termed "alkylidene")
carbene, that cyclized to the
furan 2
(ACS Catal. 2021, 11, 193.
DOI: 10.1021/acscatal.0c04713).
Xinhao Zhang of Peking University Shenzhen Graduate
School and Jiangtao Sun of Changzhou University asembled the furan 5 by coupling
the 2-pyridone 4 with the enone 3
(Angew. Chem. Int. Ed. 2021, 60, 16942.
DOI: 10.1002/anie.202104708).
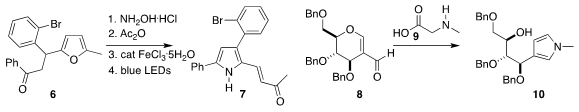
Anton S. PMID:24179643 Makarov of Perm State University rearrranged the furan 6 to the
pyrrole 7
(Org. Chem. 1240584-34-2 site Front. 2021, 8, 6553.
DOI: 10.1039/D1QO01281A).
B. V. Formula of Ir[dF(CF3)ppy]2(dtbbpy)PF6 Subba Reddy of CSIR-Indian
Institute of Chemical Technology prepared the pyrrole 10 by combining the
sugar-derived aldeyde 8 with N-methyl glycine (9)
(Tetrahedron 2021, 97, 132389.
DOI: 10.1016/j.tet.2021.132389).
Chang-Hua Ding and Bin Xu of Shanghai University coupled the ketone 11 with
the imine 12 to prepare the
pyridine 13
(Chem. Asian J. 2021, 16, 3905.
DOI: 10.1002/asia.202100983).
Yirong Zhou of the Huazhong University of Science and Technology and Fang-Lin Zhang of
the Wuhan University of Technology prepared the pyridine 16 by combining the
propargyl amine 14 with the dienal 15
(J. Org. Chem. 2021, 86, 17244.
DOI: 10.1021/acs.joc.1c02312).
Baosheng Liu of Chongqing University assembled the pyridine 19 by coupling
the dienal 18 with the alkyne 17
(Org. Lett. 2021, 23, 7883.
DOI: 10.1021/acs.orglett.1c02900).
Changkun Li of Shanghai Jiao Tong University achieved high ee in the cyclization of the
prochiral bis nitrile 20 with the alkyne 21, leading to the pyridine 22
(Angew. Chem. Int. Ed. 2021, 60, 20204.
DOI: 10.1002/anie.202105452).
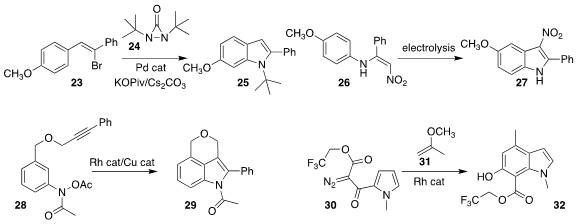
Yian Shi, also of Changzhou University, showed that the bromostyrene 23 could be cyclized to the
indole 25 using the diaziridinone 24
(Org. Lett. 2021, 23, 7561.
DOI: 10.1021/acs.orglett.1c02757).
Ashley C. Lindsay and Jonathan Sperry of the University of Auckland
employed electrolysis to cyclize the enamine 26 to the indole 27
(Org. Biomol. Chem. 2021, 19, 7903.
DOI: 10.1039/D2OB00656A).
Hai-Chao Xu of Xiamen University demonstrated an
alternative route to indoles using electrolysis
(J. Org. Chem. 2021, 86, 16001.
DOI: 10.1021/acs.joc.1c00988).
Pazhamalai Anbarasan of the Indian Institute of Technology Madras used combined Rh and Cu
catalysis to cyclize the N-acetoxy acetanilide 28 to the indole 29
(J. Org. Chem. 2021, 86, 14812.
DOI: 10.1021/acs.joc.1c01604).
Stefan France of the Georgia Institute of
Technology assembled the indole 32 by coupling the diazo ketone 30 with the enol
ether 31
(J. Org. Chem. 2021, 86, 10088.
DOI: 10.1021/acs.joc.1c00826).
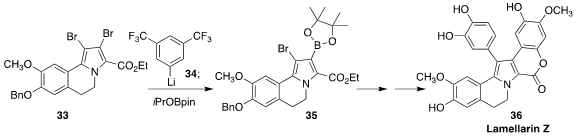
The lamellarins, exemplified by lamerallin Z (36), isolated from a variety of
marine organisms, show anti-viral and anti-cancer activity. Kentaro Okano of
Kobe University prepared 33 via a "halogen dance", then selectively
transmetalated it with 34, leading to 35, that was carried on to
36
(J. Org. Chem. 2020, 85, 8603,
DOI: 10.1021/acs.joc.0c00998;
2021, 86, 13388.
DOI: 10.1021/acs.joc.1c01505).