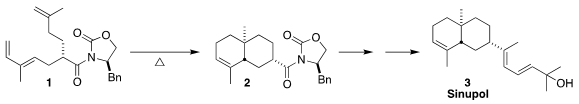
Sinupol (3), isolated from the soft coral Sinularia polydactyla,
showed moderate inhibitory activity toward protein tyrosine phosphatase 1B.
Koichiro Ota and Hiroaki Miyaoka of the Tokyo University of Pharmacy and Life
Sciences used the Evans protocol to prepare 1, securing the absolute
configuration of the final product, then cyclized 1 to 2
(Synlett 2020, 31, 1007,
DOI: 10.1055/s-0037-1610757;
Synthesis 2022, 54, 689,
DOI: 10.1055/a-1643-5729).
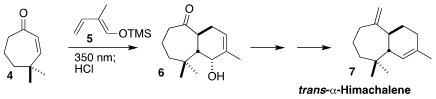
Thorsten Bach of the Technical University of Munich showed that under irradiation, the
cycloheptenone 4
was converted to its trans isomer, that readily underwent intermolecular
Diels-Alder cycloaddition with the
diene 5, leading to 6. The ketone 6 was carried on to
trans-α-himachalene (7)
(J. PMID:28322188 3,3′,5,5′-Tetrabromo-1,1′-biphenyl uses Org. Chem. 2022, 87, 4838.
DOI: 10.1021/acs.joc.2c00186). 879275-72-6 Data Sheet
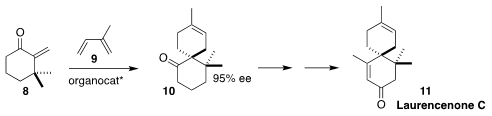
Benjamin List of the Max-Planck-Institut für Kohlenforschung devised a Binol-derived
imidodiphosphorimidate catalyst that directed the absolute sense of the cycloaddition
of the α-methylidene ketone 8 with isoprene 9, to give 10.
The ketone 10 was carried on to laurencenone C (11)
(J. Am. Chem. Soc. 2022, 144, 6703.
DOI: 10.1021/jacs.2c01971).
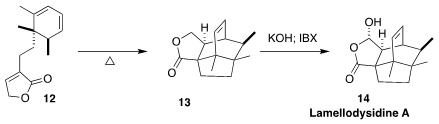
Lamellodysidine A (14), isolated from the marine sponge Lamellodysidea herbacea
collected in Indonesia, showed cytotoxic and antimicrobial activity. En route to
14, Kazuyuki Sugita of Hoshi University cyclized the triene 12 to the
lactone 13
(Org. Lett. 2022, 24, 921.
DOI: 10.1021/acs.orglett.1c04289).
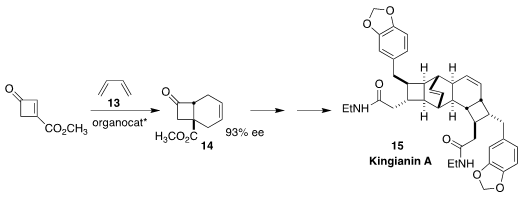
Kingianin A (15), isolated from the bark of Endiandra kingiana Gamble, a tree
collected in the rain forest of Pahang State in Malaysia, showed selective
cytotoxicity toward certain lung cancer cell lines. Jinbo Zhao of the Changchun
University of Technology and Qin Chen and Ping Lu of Fudan University devised an
oxazaborolidinium catalyst that mediated the absolute sense of the addition of
the cyclobutenone 12 to butadiene 13, leading to the ester 14 and thus to 15
(Synthesis 2022, 54, 1977.
DOI: 10.1055/s-0041-1737339).
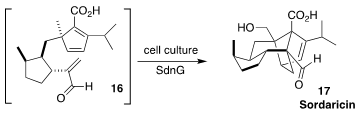
The biosynthesis of sordaricin (17), the precursor to the antifungal sordarin,
had long been known to proceed via cyclization of the triene 16. K. N. Houk and
Yi Tang of UCLA have now identified the "Diels-Alderase" enzyme that mediates
the cycloaddition, allowing the efficient production of 17 in cell culture
(Nature Commun. 2022, 13, 2568.
DOI: 10.1038/s41467-022-30288-6).