Carbon-carbon bond formation is fundamental to all of organic chemistry. PMID:28440459 The emphasis this week is on recently-developed useful transformations that are easily scalable. Notably, these transformations telescope two or more synthesis steps into one-pot procedures.
Usually, to homologate an alcohol such as 1 to the corresponding nitrile 2 one would expect to first convert the alcohol into a leaving group. Nasser Iranpoor and Habib Firouzabadi of Shiraz University, Iran, have shown (J. 1308384-31-7 supplier Org. Chem. 2004, 69, 2562.DOI: 10.1021/jo035238v)that on exposure to a combination of Ph3P, Bu4NCN and DDQ, 1 is converted directly to 2. The stereochemical outcome was not mentioned, but one would expect the reaction to proceed with net inversion, as illustrated.

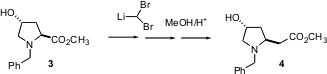
Often, esters are homologated by one carbon using the diazomethane-basedArndt-Eistert procedure. 170853-04-0 Chemscene Kowalski homologation, addition of the inexpensive dibromomethane followed by α-elimination, is a more scalable alternative. Timothy Gallagher of the University of Bristol recently reported (J. Org. Chem. 2004, 69, 4849.DOI: 10.1021/jo049562h)the use of Kowalski homologation to prepare β-amino esters from α-amino esters, including the conversion of 3 to 4. Note that the transformation can be carried out without protection of the OH, and that it proceeds without loss of stereochemical integrity.
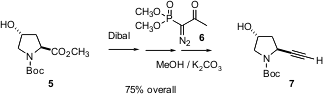
A third scalable method for one-carbon homologation, the conversion of an ester 5 to the terminal alkyne7, was reported (Tetrahedron Lett. 2004, 45, 5597.DOI: 10.1016/j.tetlet.2004.05.139)by Kevin Hinkle of GlaxoSmithKline in Research Triangle Park, NC. The ester is reduced withDibal, the intermediate is quenched with methanol, and the solution of liberated aldehyde is treated with K2CO3 and the reagent 6 according to the Ohira procedure. Weinreb esters also work well with this protocol. The conversion was reported to work in 72% yield starting with 27 g of5. Note that 6 has a DSC exotherm at about 70°C, so caution should be exercised in handling it.
Usually, one would think of alkylating a ketone such as 8 with an alkyl halide to homologate it to10. Often, aldol condensation with an aldehyde will proceed in higher yield, but this then requires three steps, aldol addition, dehydration, and hydrogenation, to reach10. Now Kiyotomi Kaneda of Osaka University has described (J. Am. Chem. Soc. 2004, 126, 5662.DOI: 10.1021/ja049181l)a Ru-grafted hydrotalcite catalyst that will mediate four steps, oxidation of 9 to the aldehyde, aldol condensation, dehydration and hydrogenation, in a single pot. The homologation also works with alcohols and arylacetonitriles.
![]()
In Memoriam: We note the untimely passing in December 2004 of Dr. Conrad Kowalski, a world-class contributor to new methods development and to scientific administration. We will miss both his chemical insight and his ready wit.