Bruce H. Lipshutz of the University of California, Santa Barbara developed
surfactants that allowed the aqueous enzymatic
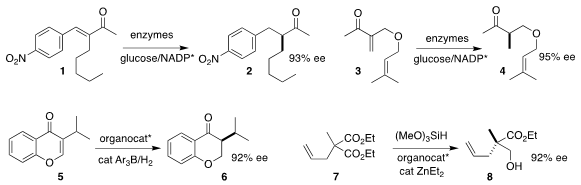
reduction of the enone
1 to the ketone 2
(Chem. Commun. 2021, 57, 11847.
DOI: 10.1039/D1CC04774D).
Jörg Pietruszka of Heinrich-Heine-Universität Düsseldorf also used an enzymatic system to reduce
the Morita-Baylis-Hillman derived ether 3 to the useful five-carbon chiron 4
(Angew. Chem. Int. Ed. 2021, 60, 16700.
DOI: 10.1002/anie.202103406).
Xiangqing Feng, Wei Meng and Haifeng Du of the University of Chinese Academy of Sciences used
a chiral phosphoric acid-derived frustrated Lewis pair to catalyze the enantioselective reduction
of the chromone 5 to the
cyclic ketone 6
(Org. PMID:24377291 Lett. MC-Val-Cit-PAB Order 2021, 23, 8565.
DOI: 10.1021/acs.orglett.1c03286).
Zhongxing Huang of the University of Hong Kong effected the enantioselective reduction of the prochiral
malonate 7 to the alcohol 8
(Nature Chem. 2021, 13, 634.
DOI: 10.1038/s41557-021-00715-0).
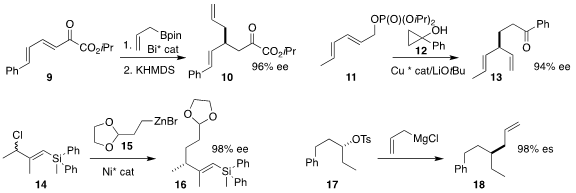
Xin Li of Nankai University showed that 1,2-allylation of the enone 9 followed by
oxy-Cope rearrangement led to the α-keto ester
10 in high ee
(Org. Lett. Mal-PEG1-acid Chemical name 2021, 23, 9128.
DOI: 10.1021/acs.orglett.1c03453).
Liang Yin of the Shanghai Institute of Organic Chemistry coupled the cyclopropanol 12
with the crotyl phosphate 11 to give the ketone 13
(Angew. Chem. Int. Ed. 2021, 60, 26351.
DOI: 10.1002/anie.202110709).
Martin Oestreich of the Technische Universität Berlin showed that the
alkenyl silane 16 could be prepared in high
ee by coupling the racemic chloride 14 with the organozinc 15
(Angew. Chem. Int. Ed. 2021, 60, 13652.
DOI: 10.1002/anie.202102233).
Erik J. Alexanian of the University of North Carolina
underlined the value of commerical allyl magnesium chloride as a nucleophile,
showing that it coupled with the secondary tosylate 17 to give the
allylated product 18
with inversion of absolute configuration
(Org. Lett. 2021, 23, 7215.
DOI: 10.1021/acs.orglett.1c02616).
Rylan J. Lundgren of the University of Alberta opened racemic isoprene monoepoxide 20 with
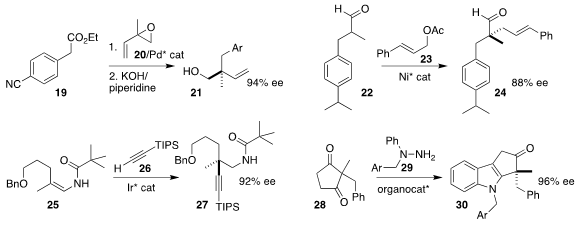
the α-aryl ester 19, leading after decarboxylation to the alcohol 21
(Angew. Chem. Int. Ed. 2021, 60, 26495.
DOI: 10.1002/anie.202110525).
Hong-Cheng Shen of the University of Science and Technology of China assembled the α-quaternary
aldehyde 24 by alkylating the aldehyde 22 with cinnamyl acetate 23
(ACS Catal. 2021, 11, 11849.
DOI: 10.1021/acscatal.1c03449).
Bi-Jie Li of Tsinghua University prepared the the α-quaternary
alkyne 27 by adding the alkyne 26 to the alkenyl amide 25
(J. Am. Chem. Soc. 2021, 143, 9639.
DOI: 10.1021/jacs.1c04493).
Garima Jindal and Santanu Mukherjee of the Indian Institute of
Science Bangalore achieved high ee in the desymmetrizing
Fischer indolization
of the prochiral cyclopentanedione 28 with the hydrazine 29 to give the indole 30
(Angew. Chem. Int. Ed. 2021, 60, 9086.
DOI: 10.1002/anie.202017268).
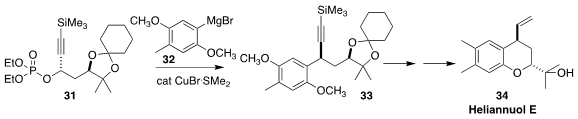
Heliannuol E (34) was isolated from the common sunflower Helianthus annuus.
Narihito Ogawa of Meiji University established the absolute configuration of the
alkylated stereogenic center of 34 by coupling the propargylic phosphate 31 with
the aryl Grignard reagent 32, leading to 33
(Synlett 2021, 32, 2071.
DOI: 10.1055/s-0040-1719844).