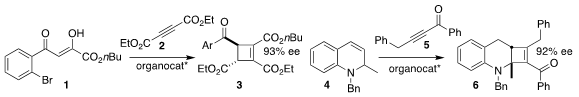
Arnaud Voituriez of the Université Paris-Saclay used a cyclic phosphine to
mediate the assembly of the
cyclobutene
3 by the addition of the β-diketone 1
to the acetylene dicarboxylate 2
(Adv. Synth. Catal. 2021, 363, 4805.
DOI: 10.1002/adsc.202100664).
Xiao-Chen Wang of Nankai University showed that a bis-borane catalyst effected both alkene
migration and addition to the alkynyl ketone 5, leading to the cyclobutene 6
(Angew. Price of 2,4-Dichloro-5-fluoro-6-methylpyrimidine Chem. Int. Ed. 1370633-67-2 Formula 2021, 60, 17185.
DOI: 10.1002/anie.202106168).
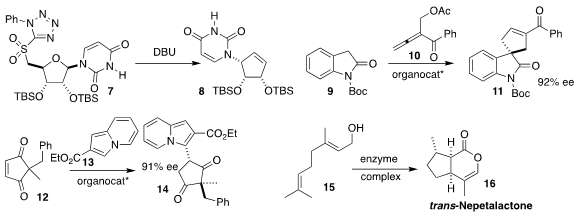
Natsuhisa Oka and Kaori Ando of Gifu University rearranged the
nucleoside-derived tetrazole 7 to the
cyclopentene 8
(J. Org. PMID:28739548 Chem. 2021, 86, 16684.
DOI: 10.1021/acs.joc.1c01940).
Yixin Lu of the National University of Singapore used an amidophosphine
to promote the addition of the
oxindole
9 to the allenyl ketone 10, leading to
the cyclopentene 11
(Org. Chem. Front. 2021, 8, 4485.
DOI: 10.1039/D1QO00669J).
Qijian Ni and Xiaoxiao Song of Anhui Normal University showed that a chiral phosphoric was directed the
conjugate addition of the
indolizine
13 to the prochiral diketone 12, to give 14
(Org. Lett. 2021, 23, 9548.
DOI: 10.1021/acs.orglett.1c03780).
Yi Tang of UCLA employed a cell-free enzyme complex
to convert geraniol 15 to the important insect repellent nepetalactone 16
(ACS Catal. 2021, 11, 9898.
DOI: 10.1021/acscatal.1c02267).
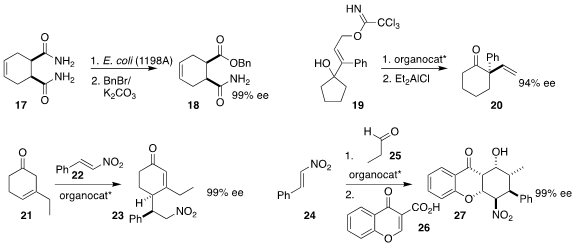
Yu-Fei Ao of the Institute of Chemistry of the Chinese Academy of Sciences
used a modified strain of E. coli to selectively
hydrolyze the prochiral
bisamide 17, leading to the monoester 18
(Adv. Synth. Catal. 2021, 363, 4538.
DOI: 10.1002/adsc.202100597).
Masahiro Terada of Tohoku University used a chiral bisphosphoric acid in
combination with a boronic acid to cyclize the prochiral tertiary alcohol 19 to
the corresponding spiro epoxide, that was further rearranged to the
cyclohexanone 20
(Tetrahedron 2021, 98, 132412.
DOI: 10.1016/j.tet.2021.132412).
Gonghua Song and Jinxing Ye of
the East China University of Science and Technology showed that with a diamine
catalyst, addition of the deconjugated
cyclohexenone
21 to the nitro alkene 22 led to the γ-adduct 23
(Org. Chem. Front. 2021, 8, 4758.
DOI: 10.1039/D1QO00371B).
Xiong-Li Liu of Guizhou University used the Hayashi-Jørgensen amine to mediate the addition of
propionaldehyde 25 to the nitroalkene 24, to give an adduct that was added to
the enone 26, leading to 27
(Chem. Commun. 2021, 57, 6764.
DOI: 10.1039/D1CC02570H).
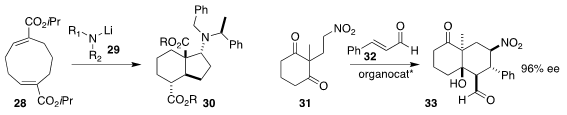
M. Rita Paleo and F. Javier Sardina of the Universidade de Santiago de Compostela prepared
the diester 30 by the addition of the amine 29 to the cyclononadiene 28
(J. Org. Chem. 2021, 86, 13684.
DOI: 10.1021/acs.joc.1c01751).
Yujiro Hayashi, also of
Tohoku University, used the Hayashi-Jørgensen amine to mediate the assembly of
33 by the addition of the diketone 31 to cinnamaldehyde 32
(Org. Lett. 2021, 23, 6654.
DOI: 10.1021/acs.orglett.1c02196).
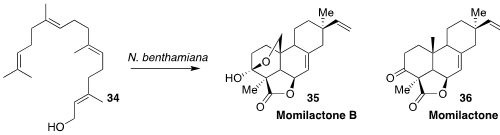
Elizabeth S. Sattely of Stanford University modified Nicotiana benthamiana to
synthesize the germination-inhibiting phytoalexin momilactone B (34). From five to
seven week old plants, 216 g of leaves yielded 3.6 mg of purified 35.
Geranylgeraniol 34 is the biosynthetic precursor to 35, but the plants were
raised on just water and fertilizer
(Nature Chem. Biol. 2021, 17, 205.
DOI: 10.1038/s41589-020-00669-3).
Momilactone B (35), the most active in this series, has not yet been prepared
by total synthesis. Pierre Deslongchamps of the Université de Sherbrooke
reported the synthesis of the simpler momilactone A (36)
(J. Org. Chem. 2002, 67, 5269.
DOI: 10.1021/jo025873l).