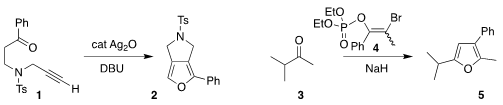
Marie-Isabelle Lannou and Geoffroy Sorin of the Université de Paris used Ag2O
to cyclize the alkyne 1 to the
furan 2
(Chem. Commun. 2022, 58, 1374.
DOI: 10.1039/D1CC06379K).
Rishikesh Narayan of the Indian Institute of Technology Goa devised
the enol phosphate 4 to convert the ketone 3 to the furan 5
(Tetrahedron 2022, 103, 132553.
DOI: 10.1016/j.tet.2021.132553).
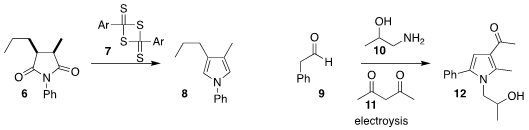
Zhen Wang of Lanzhou University used
Lawesson’s reagent (7) to convert the
succinimide 6 to the pyrrole 8
(Org. Formula of Methyl 4-bromo-2-naphthoate Chem. 6-Bromobenzo[d]isothiazole structure Front. 2022, 9, 1599.
DOI: 10.1039/D2QO00013J).
Gao-Qing Yuan of the South China University of Technology used electrolysis to promote the
assembly of the pyrrole 12 by the coupling of the amino alcohol 10, the β-diketone
11, and the aldehyde 9
(Tetrahedron Lett. PMID:35954127 2022, 90, 153615.
DOI: 10.1016/j.tetlet.2021.153615).
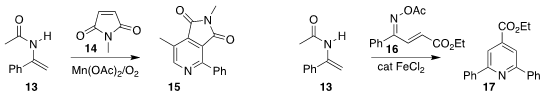
Haiqing Luo of Gannan Normal University assembled the
pyridine 15 by
combining the enamide 13 with the maleimide 14
(Adv. Synth. Catal. 2022, 364, 1683.
DOI: 10.1002/adsc.202200251).
Jindian Duan of Nanjing Tech University combined the oxime ester 16 with
13, leading to the pyridine 17
(Synlett 2022, 33, 283.
DOI: 10.1055/a-1679-7225).
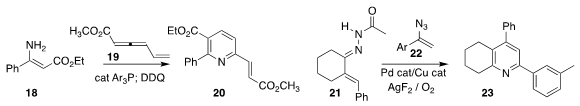
You Huang of Nankai
University used a catalytic phosphine to promote the addition of the enamine 18
to the allene 19, to give after oxidation the pyridine 20
(Adv. Synth. Catal. 2022, 364, 1879.
DOI: 10.1002/adsc.202200071).
Yingjun Zhang of Sunshine Lake PharmaCompany and Huanfeng
Jiang, also of the South China University of Technology, constructed the
pyridine 23 by combining the hydrazone 21 with the azide 22
(J. Org. Chem. 2022, 87, 159.
DOI: 10.1021/acs.joc.1c02086).
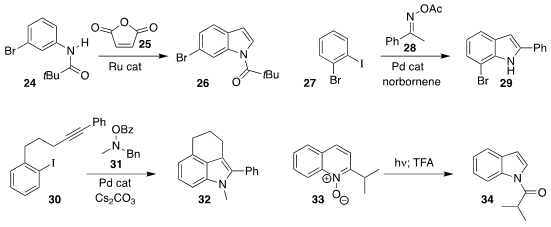
Zheyu Li, Chunran Zhang and Wenbo Ma of Chengdu University prepared the
indole 26 by adding vinylidene carbonate
25 to the anilide 24
(Adv. Synth. Catal. 2022, 364, 838.
DOI: 10.1002/adsc.202101466).
Liangbin Huang, also of the South China University of
Technology, used the Catellani protocol to construct the indole 29 from the
iodobenzene 27 and the oxime acetate 28
(Org. Lett. 2022, 24, 484.
DOI: 10.1021/acs.orglett.1c03679).
Lu Bai and Xinjun Luan of Northwest University showed selective benzyl cleavage in the
cyclization of the alkyne 30 with the hydroxylamine 31 to give the indole 32
(Angew. Chem. Int. Ed. 2022, 61, e202113820.
DOI: 10.1002/anie.202113820).
Mark D. Levin of the University of Chicago
devised conditions to excise a carbon from the quinoline N-oxide 33, leading to
the indole 34
(Science 2022, 376, 527.
DOI: 10.1126/science.abo4282).
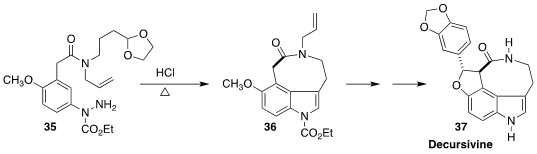
The alkaloid decursivine (37) was isolated from the creeping philodendron
Rhaphidophora decursiva. Cheon-Gyu Cho of Hanyang University devised a route to
37 based on the intramolecular
Fischer indole synthesis of
36 from the acetal 35
(Org. Lett. 2022, 24, 2873.
DOI: 10.1021/acs.orglett.2c00848).