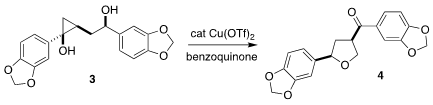The sap from the elephant tree of northern Mexico, Bursera microphylla,
was used locally as a cure-all for diseases, particularly those affecting the
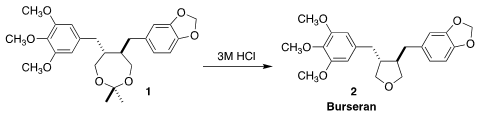
skin. Hiroshi Tanaka of the Tokyo University of Technology prepared burseran (2),
isolated from the sap, by the direct cyclization of the
acetonide 1
(Chem. Eur. 2,2-Dimethylbut-3-ynoic acid site J. PMID:24818938 2021, 27, 9422.
DOI: 10.1002/chem.202100804).
Hyperione A (4) was isolated from the Chinese medicinal herb Hypericum
chinense. Mingji Dai of Purdue University assembled the
cyclopropane 3
via the Kulinkovich reaction, then cyclized it directly to 4
(Chem. Sci. 3-(Trifluoromethyl)-1H-indazole manufacturer 2021, 12, 1311.
DOI: 10.1039/D0SC05556E).
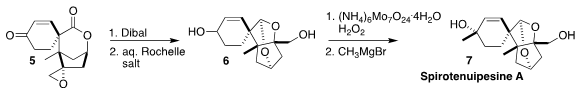
Spirotenuipesine A (7), isolated from the entomopathogenic fungus
Paecilomyces tenuipes, promoted neurotrophic factor biosynthesis in glial
cells. En route to 7, Hiroshi Imagawa of Tokushima Bunri University
converted the epoxide 5 to the tricyclic bis ether 6
(Tetrahedron Lett. 2021, 64, 152723.
DOI: 10.1016/j.tetlet.2020.152723).
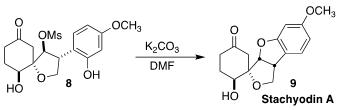
Stachyodin A (9), isolated from the roots of the Chinese medicinal
shrub Indigofera stachyode, showed anti-inflammatory activity. Hisanaka
Ito of the Tokyo University of Pharmacy and Life Sciences cyclized the phenol
8 directly to 9
(Org. Lett. 2021, 23, 3864.
DOI: 10.1021/acs.orglett.1c00998).
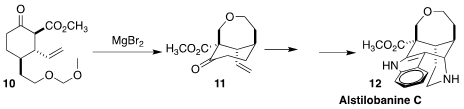
Alstilobanine C (12), isolated from the Malaysian tree Alstonia
angustiloba, is a selective inhibitor of butyrylcholinesterase. Jieping Zhu of
the Ecole Polytechnique Fédérale de Lausanne constructed the
oxepane ring of
12 by the cyclization of 10 to 11
(Angew. Chem. Int. Ed. 2021, 60, 12392.
DOI: 10.1002/anie.202103580).
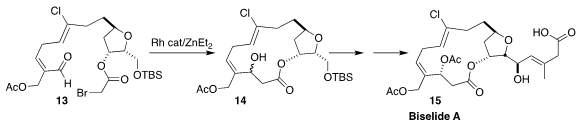
Biselide A (15), isolated from the Okinawan ascidian Didemnidae
sp., showed significant preferential toxicity toward cancer cell lines. Robert
Britton of Simon Fraser University assembled 15 via the intramolecular
Reformatsky cyclization of the
bromacetate 13 to the macrolide 14
(Chem. Sci. 2021, 12, 5534.
DOI: 10.1039/D0SC06223E).