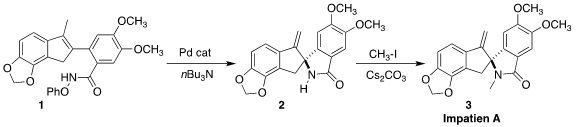
Impatien A (3) was isolated from the Corydalis impatien, a
flower of south China that is an important component in traditional Chinese
medicine. Cyclopropanecarbaldehyde Order Donald A. Watson of the University of Delaware established the spiro
ring fusion of 3 by the Pd-catalyzed cyclization of the phenoxy amide
1 to the
lactam 2 (Org. PMID:24367939 Lett. 2021, 23, 7285.
DOI: 10.1021/acs.orglett.1c02767).
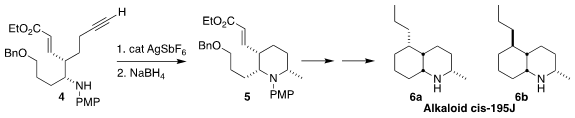
The alkaloid cis-195J (6a or 6b) was isolated from the
skin extract of the neotropical dendrobatid frog Mantella betsileo.
Christoph Schneider of the Universität Leipzig individually prepared 6a
and 6b from the trisubstituted
piperidine 5,
assembled by the cyclization of the alkyne 4 followed by reduction. Biosynthetic
considerations suggest that the natural product is 6b, but confirmation
of this will wait on comparison with the natural alkaloid
(J. 101364-27-6 Data Sheet Org. Chem. 2021, 86, 11960.
DOI: 10.1021/acs.joc.1c01346).
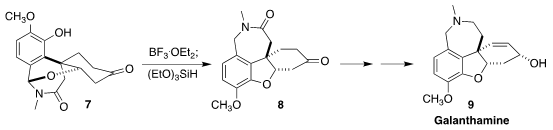
Galanthamine (9), isolated from the common daffodil, has found
widespread application in the treatment of dementia. Yu-Ming Zhao of Shaanxi
Normal University developed a route to 9 by way of
the lactam 8,
prepared by the acid-mediated rearrangement of the hemiaminal 7, followed
by reduction
(Org. Lett. 2021, 23, 9659.
DOI: 10.1021/acs.orglett.1c03943).
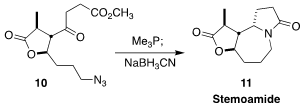
Stemoamide (11) was isolated from Stemona tuberosa, a flowering
plant native to China, India, southeast Asia, and New Guinea. Yefeng Tang of
Tsinghua University achieved high diastereoselectivity in the reduction of the
imine derived from 10, leading directly, after spontaneous lactam
formation, to 11
(Angew. Chem. Int. Ed. 2021, 60, 14545.
DOI: 10.1002/anie.202102614).
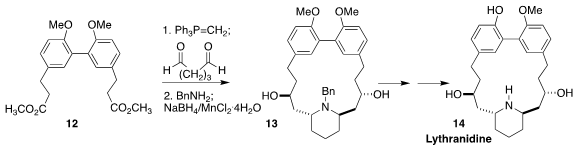
The macrocyclic alkaloid lythranidine (14) was isolated from the
Japanese perennial Lythrum anceps Makino. Michael S. Sherburn of
Australian National University carried the diester 12 on to the
17-membered ring dienone, condensed that with benzylamine, then reduced the
diketone so produced to the diol 13. Selective
deprotection then
completed the synthesis of 14
(Angew. Chem. Int. Ed. 2021, 60, 18561.
DOI: 10.1002/anie.202107524).
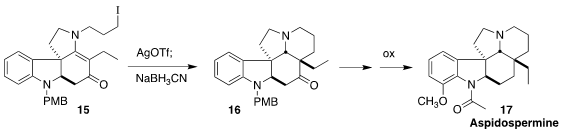
Aspidospermine (17), isolated from the tree Aspidosperma of
tropical South America, has adrenergic blocking activity. Yongxiang Liu of
Shenyang Pharmaceutical University assembled the pentacyclic ketone 16 by
the Ag-mediated cyclization of the iodide 15, followed by reduction
(Org. Lett. 2021, 23, 6471.
DOI: 10.1021/acs.orglett.1c02287).