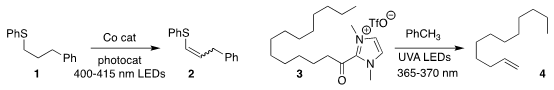
Chen Xu of the Southern University of Science and Technology and Zheng Huang
of the Shanghai Institute of Organic Chemistry effected the acceptorless
photochemical dehydrogenation of the sulfide 1 to the alkene 2
(J. Am. Chem. Soc. Fmoc-D-Isoleucine site 2021, 143, 16470.
DOI: 10.1021/jacs.1c05479).
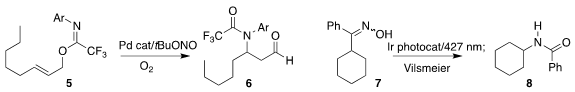
Matthew N. Hopkinson, now at the University of Newcastle, showed
that under irradiation, the carboxylic acid derivative 3 was converted to the
terminal alkene 4
(Tetrahedron 2021, 100, 132497.
DOI: 10.1016/j.tet.2021.132497).
Jian-Ping Qu of Nanjing Tech University and Yan-Biao Kang of the University
of Science and Technology of China rearranged the allylic imidate 4 to the
β-amino aldehyde 6
(Org. Lett. BuyMethyl 5-fluoro-2-methoxyisonicotinate 2021, 23, 9273.
DOI: 10.1021/acs.orglett.1c03619).
Tomislav Rovis of Columbia University showed that visible light irradiation converted the
oxime to the less stable geometric isomer,
that on exposure to the Vilsmeier reagent was carried on the amide 8
(J. Am. Chem. PMID:23891445 Soc. 2021, 143, 21211.
DOI: 10.1021/jacs.1c10148).
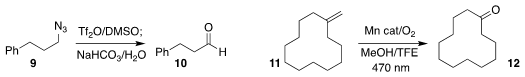
Hiroki Tanimoto of Toyama University converted the azide 9 directly to the
aldehyde 10
(Chem. Commun. 2021, 57, 8738.
DOI: 10.1039/D1CC02770K).
Jianliang Xiao of the University of Liverpool used a manganese catalyst and visible
light to oxidize the alkene 11 to the ketone 12
(J. Am. Chem. Soc. 2021, 143, 10005.
DOI: 10.1021/jacs.1c05757).
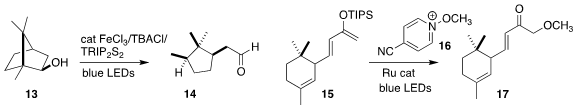
Peng Hu of Sun Yat-Sen
University achieved high diastereocontrol in the cleavage of the secondary
alcohol 13 to the aldehyde 14
(Org. Lett. 2021, 23, 8413.
DOI: 10.1021/acs.orglett.1c03137).
Guillaume Dagousett of Université Paris-Saclay used the reagent 16 to directly
methoxylate the silyl enol ether 15, leading to 17
(Org. Lett. 2021, 23, 8926.
DOI: 10.1021/acs.orglett.1c03444).
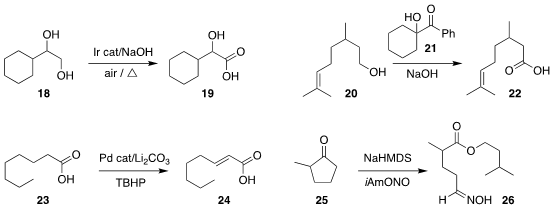
Xin Xu and Tao Tu of Fudan University showed that the diol 18 could be
oxidized to the α-hydroxy acid 19
(ACS Catal. 2021, 11, 12833.
DOI: 10.1021/acscatal.1c04354).
Hongbin Zhang of Yunnan University used the commercial reagent 21 to oxidize the alcohol
20 to
the acid 22
(Org. Lett. 2021, 23, 6648.
DOI: 10.1021/acs.orglett.1c02188).
Jin-Quan Yu of Scripps-La Jolla oxidized the acid 23 directly to the
α,β-unsaturated acid 24
(Science 2021, 374, 1281.
DOI: 10.1126/science.abl3939).
Ullrich Jahn of the Czech Academy of Sciences effected the regioselective
cleavage of the ketone 25 to the oxime ester 26
(J. Org. Chem. 2021, 86, 11608.
DOI: 10.1021/acs.joc.1c01169).
Shenlin Huang of Nanjing Forestry University reported a parallel investigation
(Org. Lett. 2021, 23, 6525.
DOI: 10.1021/acs.orglett.1c02327).
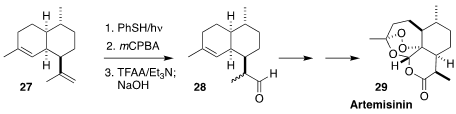
The use of artemisinin (29) in malaria therapy has made the development of a
practical synthesis particularly urgent. En route to 29, Janine Cossy of ESPCI
Paris and Zacharias Amara of HESAM Université described the conversion of
amorphadiene 27 to the aldehyde 28
(Org. Lett. 2021, 23, 5593.
DOI: 10.1021/acs.orglett.1c00636).