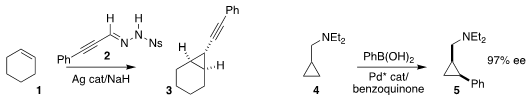
Xihe Bi of Northeast Normal University showed that the diazo alkane generated
in situ from the sulfonylhydrazide 2 could with a silver catalyst be added to
the alkene 1, to give the
cyclopropane 3
(Chem. 885270-86-0 Order Commun. 2022, 58, 3485.
DOI: 10.1039/D2CC00099G).
Matthew J. Gaunt of the University of Cambridge effected enantioselective metalation of
the prochiral amine 4, leading to the cyclopropane 5
(J. Am. Chem. Soc. 2022, 144, 3939.
DOI: 10.1021/jacs.1c11921). 3,3′-Oxybis(propan-1-ol) custom synthesis
Stephen P. Fletcher of the University of Oxford achieved high
enantioselectivity in the coupling of the prochiral
cyclobutene 6
with salicylaldehyde 7 to give the
cyclobutane 8
(Chem. Sci. 2022, 13, 236,
DOI: 10.1039/D1SC06035J;
Chem. Sci. 2022, 13, 1177,
DOI: 10.1039/D1SC90265B).
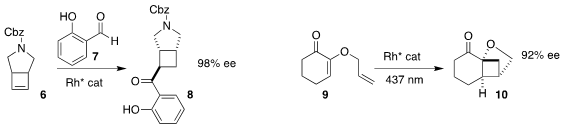
Thorsten Bach of the Technische Universität München used a Rh catalyst to direct the
photocyclization of the α-alkoxy enone 9 to the cyclobutane 10
(Chem. Sci. 2022, 13, 2378.
DOI: 10.1039/D2SC00113F). PMID:35991869
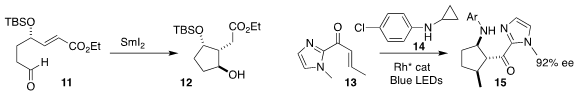
Pei Tang of Sichuan University and Fener Chen of Fudan University employed
stoichiometric SmI2 to cyclize the aldehyde 11
to the alcohol 12
(Chem. Commun. 2022, 58, 6000.
DOI: 10.1039/D2CC01737G).
Alberto Fraile and José Aleman of the Universidad Autónoma de Madrid assembled the amine
15 by coupling the acyl imidazole 13 with the cyclopropyl amine 14
(Chem. Commun. 2022, 58, 1334.
DOI: 10.1039/D1CC05867C).
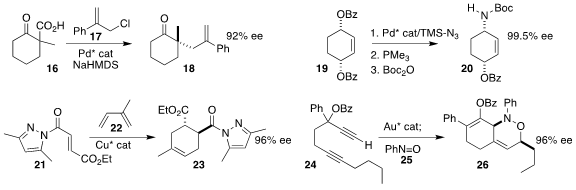
Aaron Aponik of the University of Florida coupled the racemic acid 16 with
the allylic chloride 17, leading to the
cyclohexanone 18 in high ee
(ACS Catal. 2022, 11, 14842.
DOI: 10.1021/acscatal.1c04546).
Thomas E. La Cruz of Bristol Myers Squibb also achieved high ee in the conversion of
the prochiral bis-benzoate 19 to the protected cyclohexyl amine 20
(J. Org. Chem. 2022, 87, 1996.
DOI: 10.1021/acs.joc.1c01162).
Kazuaki Ishihara of Nagoya University developed a Cu catalyst that mediated the
Diels-Alder cycloaddition
of the acyl pyrazole 21 with isoprene 22, leading to the ester 23
(Synlett 2022, 33, 585.
DOI: 10.1055/a-1750-8481).
Alireza Ariafard of the University of Tasmania and Philip Wai Hong
Chan of Monash University used a gold catalyst to mediate the coupling of the
diyne 24 with nitrosobenzene 25, leading to the
cyclohexene 26
(ACS Catal. 2022, 12, 7288.
DOI: 10.1021/acscatal.2c01680).
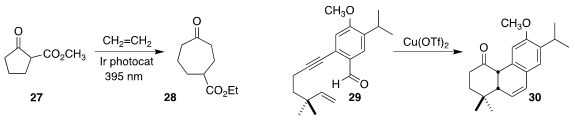
Dirk M. Guldi of Friedrich-Alexander-Universität Erlangen-Nürnberg and Frank Glorius
of the Westfälische Wilhelms-Universität Münster effected the ring expansion of the
cyclopentanone 27 to the
cycloheptanone 28
(Angew. Chem. Int. Ed. 2022, 61, e202112695.
DOI: 10.1002/anie.202112695).
Chang Ho Oh of Hanyang University used Cu(OTf)2 to catalyze
the conversion of the enyne 29 to the tricyclic ketone 30
(Synlett 2022, 33, 983.
DOI: 10.1055/a-1801-4344).
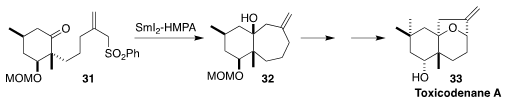
Toxicodenane A (33), isolated from the dried resin of the lacquer tree
Toxicodendron vernicifluum, showed some protective activity against
lipid-induced toxicity in cultured renal proximal tubular cells. Keisuke
Nishikawa and Yoshiki Morimoto of Osaka City University established the
cycloheptane ring of
33 by the Barbier-type cyclization of the keto sulfone 31
to the tertiary alcohol 32
(Org. Lett. 2022, 24, 531.
DOI: 10.1021/acs.orglett.1c03924).