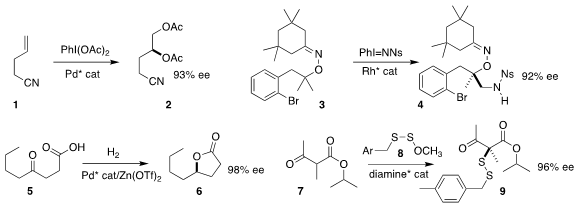
Guosheng Liu of the Shanghai Institute of Organic Chemistry devised the enantioselective
Pd-catalyzed diacetoxylation of the alkene 1 to the bis-acetate 2
(Nature Catal. 2021, 4, 172.
DOI: 10.1038/s41929-021-00574-5).
Bingxian Liu and Junbiao Chang of Henan Normal
University and Xingwei Li of Shaanxi Normal University developed the
enantioselective Rh-catalyzed
amination of the gem-dimethyl group of 3, leading
to the sulfonamide 4
(Angew. Chem. PMID:25269910 Int. Ed. 2021, 60, 8396.
DOI: 10.1002/anie.202014080).
Jingchao Chen and Baomin Fan of Yunnan Minzu University used a Pd catalyst to reduce the γ-keto
acid 5 to the γ-lactone 6
(Chem. Asian. J. 2021, 16, 1229.
DOI: 10.1002/asia.202100244).
Sanzhong Luo of Tsinghua University used a diamine catalyst to mediate the combination of the β-ketoester
7 with the disulfide 8 to give the disulfide 9
(Angew. Formula of 1451091-01-2 Chem. Int. Ed. 2090040-33-6 uses 2021, 60, 10971.
DOI: 10.1002/anie.202101569).
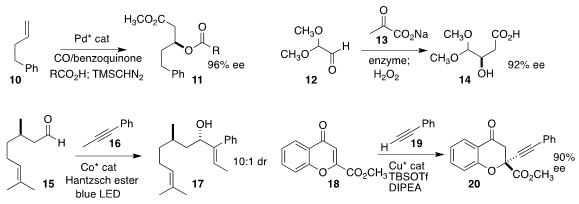
Guosheng Liu also developed the enantioselective Pd-catalyzd oxidative carbonylation of
the terminal alkene 10 leading to the acylated β-hydroxy ester 11
(Angew. Chem. Int. Ed. 2021, 60, 14881.
DOI: 10.1002/anie.202104252).
Pere Clapés of ICIQ optimized the enzymatic
homologation of the aldehyde 12 with
13 to the β-hydroxy acid 14
(ACS Catal. 2021, 11, 4660.
DOI: 10.1021/acscatal.1c00210).
Ji-Bao Xia of the Lanzhou Institute of Chemical Physics achieved high regioselectivity and diastereoselectivity in the
coupling of the enantiomerically-pure aldehyde 15 with the alkyne 16 to give the
allylic alcohol 17
(J. Am. Chem. Soc. 2021, 143, 7306.
DOI: 10.1021/jacs.1c03527).
Anita E. Mattson of Worcester Polytechnic Institute assembled the tertiary ether
20 by adding the alkyne 19 to the ester 18
(ACS Catal. 2021, 11, 6325.
DOI: 10.1021/acscatal.1c01095).
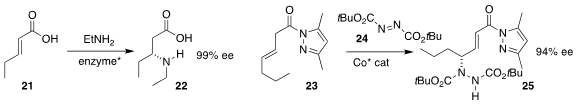
Bian Wu of the Institute of Microbiology of the Chinese Academy of Sciences
optimized a lyase for the amination of 21 to give the β-amino acid 22
(Nature Catal. 2021, 4, 364.
DOI: 10.1038/s41929-021-00604-2).
Abing Duan of Hunan University and Shu-Yu Zhang of
Shanghai Jiao Tong University coupled the unsaturated amide 23 with the
azodicarboxylate 24, leading to the γ-amino amide 25
(Org. Lett. 2021, 23, 25.
DOI: 10.1021/acs.orglett.0c03522).
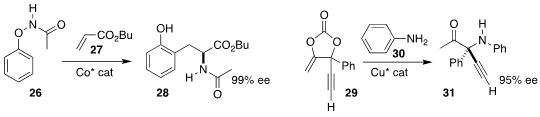
Nicolai Cramer of EPFL devised the enantioselective ortho coupling of 26 with
the acrylate 27, leading to the γ-amino ester 28
(Angew. Chem. Int. Ed. 2021, 60, 655.
DOI: 10.1002/anie.202011140).
Zhi Zhou and Wei Yi of Guangzhou Medical University
(ACS Catal. 2021, 11, 2279.
DOI: 10.1021/acscatal.0c04777),
Yu Liu of the Changchun University of Technology and Junliang Zhang of Fudan University
(Chem. Sci. 2021, 12, 8241.
DOI: 10.1039/D1SC01337H)
and Fen Wang and Professor Li of Shaanxi Normal University
(ACS Catal. 2021, 11, 6692.
DOI: 10.1021/acscatal.1c01615)
described parallel investigations. Wusheng Guo of the Xi’an Jiaotong University assembled
the γ-quaternary amine 31 by coupling aniline 30 with the carbonate 29
(J. Am. Chem. Soc. 2021, 143, 7629.
DOI: 10.1021/jacs.1c03182).
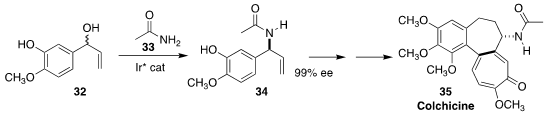
Many derivatives of colchicine (35) have been prepared in pursuit of improved
anti-cancer activity. Yu-Rong Yang of the Kunming Institute of Botany developed
a seven-step route to 35, a key transformation of which was the enantioselective
preparation of the amide 34 by the Ir-catalyzed coupling of the racemic allylic
alcohol 32 with acetamide 33
(Org. Lett. 2021, 23, 2731.
DOI: 10.1021/acs.orglett.1c00638).